fibröse Dysplasie der Kalotte

Fibrous
dysplasia for radiologists: beyond ground glass bone matrix. Age-related changes in fibrous dysplasia (FD). CT of the head on the same patient at age of 6 (a), 7 (b) and 14 years (c). Diffuse FD involvement with homogenous “ground glass” appearance (green arrows), which demonstrates the tendency to develop cystic lesions and become more heterogeneous with time (green arrows)

Fibrous
dysplasia for radiologists: beyond ground glass bone matrix. Brain compression in craniofacial fibrous dysplasia (FD). A patient with known right parietal bone FD (a) develops an aneurysmal bone cyst causing mass effect on adjacent brain (b–e)

Bild wie bei
Fibröser Dysplasie der Schädelkalotte links temporal: Milchglasartige Läsion mit lytischer Zone und sklerosiertem Kern. Nicht histologisch bestätigt, aber gut passend.
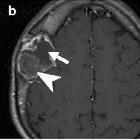
Imaging of
skull vault tumors in adults. Fibrous dysplasia. T2WI (a), post-contrast T1WI (b), and NECT (c). Expansile lesion with distinct lytic-cystic component (arrows) and “ground-glass” fibrous tissue (arrowheads) clearly depicted on NECT, deeply T2-hypointense, which enhances heterogeneously

McCune-Albright
syndrome. Radiographic appearance of fibrous dysplasia (FD). A) A proximal femur with typical ground glass appearance and shepherd"s crook deformity in a 10-year-old child is shown. B) The appearance of FD in the femur of an untreated 40-year-old man demonstrates the tendency for FD to appear more sclerotic with time C) The typical ground glass appearance of FD in the craniofacial region on a CT image of a 10-year-old child is shown. The white arrows indicate the optic nerves, which are typically encased with FD. D) A CT image in a 40-year-old woman demonstrates the typical appearance of craniofacial FD in an older person, with mixed solid and "cystic" lesions. The Hounsfield Unit measurements of "cystic" lesions are quite useful in distinguishing soft tissue "cystic" lesions from true fluid-filled cysts, which are much more uncommon and tend to behave aggressively with rapid expansion and compression of vital structures. E-G) Bone Scintigraphy in FD. Representative 99Tc-MDP bone scans which show tracer uptake at affected skeletal sites, and the associated skeletal disease burden score (see ref. Collins, 2005) are shown. E) A 50-year-old woman with monostotic FD confined to a single focus involving contiguous bones in the craniofacial region. F) A 42-year-old man with polyostotic FD shows the tendency for FD to be predominantly (but not exclusively) unilateral, and to involve the skull base and proximal femur. G) A 16-year-old boy with McCune-Albright syndrome and involvement of virtually all skeletal sites (panostotic) is shown [65].

Fibrous
dysplasia for radiologists: beyond ground glass bone matrix. CT in fibrous dysplasia (FD). CT imaging is the modality of choice and superior to radiographs in delineating morphological changes in bone. Radiographs are not recommended for diagnostic purposes or for the characterisation of craniofacial lesions. a, b Radiographs of the head show evidence of FD (red arrow). c, d CT of the head on the same patient delineates lesions and demonstrates relationship between lesions and neuronal, vascular and soft tissue structures (green arrow)

Fibrous
dysplasia for radiologists: beyond ground glass bone matrix. The location-based difference in appearance of fibrous dysplasia (FD) lesions. a Craniofacial FD demonstrates dense, sclerotic lesions (green arrow). b Lesions in the long bones and axial skeleton are typically lucent. There is a typical lesion in the proximal femur with characteristic lucent ground glass appearance and shepherd’s crook deformity (blue arrow). c Mixed radiolucent/lytic FD lesions in the skull
fibröse Dysplasie der Kalotte
Siehe auch:

 Assoziationen und Differentialdiagnosen zu fibröse Dysplasie der Kalotte:
Assoziationen und Differentialdiagnosen zu fibröse Dysplasie der Kalotte:



