urämische Enzephalopathie



Uremic encephalopathy (UE) is an acquired toxic syndrome characterized by delirium in patients with untreated or inadequately treated end-stage renal disease. Uremic encephalopathy is often associated with lethargy and confusion in the acute phase, which can progress to seizures, coma, or both in the chronic phase. Several neurochemical alterations have been reported in the acute and chronic phases of uremic encephalopathy, including alterations in water transport and cerebral edema, alterations in the blood-brain barrier, and changes in cerebral metabolism.
Epidemiology
There are very few studies dealing with the epidemiology of the encephalopathic aspect of renal dysfunction. One study investigated cognitive impairment in patients on haemodialysis and found that among 374 patients on haemodialysis, 55 years or older, only 12.7% were completely cognitively intact. Almost 14% demonstrated mild cognitive impairment, 36.1% demonstrated moderate cognitive impairment, and 37.3% demonstrated severe cognitive impairment . Uremic retention solutes, anemia and hyperparathyroidism may play distinct roles in the pathogenesis of uremic encephalopathy .
Clinical presentation
Uremic encephalopathy presents as a complex syndrome which varies in its presentation from mild sensory clouding to delirium and coma, occasionally associated with myoclonus, asterixis, and seizures .
Pathology
Etiology
Animal studies have demonstrated impressive activations of biogenic amine expressing cell groups, stress-sensitive areas, and cell groups involved in the regulation of water and electrolyte homeostasis, as well as central autonomic cell groups, in surgically and medically induced renal failure in rats . Data reported indicated that the acute uremic state is an important factor that influences a variety of neurochemicals such as the biogenic amines (norepinephrine, epinephrine, histamine, and 5-hydroxytryptamine(5-HT)), as well as various brain areas such as stress-sensitive forebrain areas, neuronal cell groups involved in the regulation of water and electrolyte homeostasis, and central autonomic cell groups . Recent animal studies comparing acute uremic encephalopathy with hepatic encephalopathy have demonstrated an increase in brain inflammation in conjunction with an increase in vascular permeability in uremic encephalopathy . Local kidney injury may activate cytokines that cross the blood-brain barrier or activate other messengers that contribute to neuronal dysfunction. Alternatively, the retention of uremic solutes may trigger both the inflammatory reaction and neuronal dysfunction .
Case reports and studies in humans have reported a number of biochemical changes in acute and chronic uremic encephalopathy, including alterations in water transport and brain edema, disturbances of the blood-brain barrier, and changes in cerebral metabolism . Anemia, hyperparathyroidism and brain calcium concentrations have also been implicated in uremic encephalopathy .
Macroscopic and microscopic features
Non-specific neuropathologic abnormalities have been described, including cerebral atrophy, gliosis, and foci of perivascular necrosis with accumulation of macrophages. Alzheimer type II astrocytes are often prominent. Patients may also develop changes of hypertensive encephalopathy .
Radiographic features
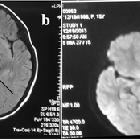
UE is a syndrome in which the subcortical gray and white matter, midbrain and mesial temporal lobes become edematous due to their exquisite sensitivity to metabolic alterations which is an inherent vulnerability related to the arterial perforators which supply these areas. The imaging features are thus consistent with cytotoxic edema, localized in those areas.
CT
Uremic encephalopathy on CT typically presents as confluent bilateral hypodensity involving the basal ganglia, thalamus and midbrain. The anatomical boundaries between the deep subcortical gray matter typically appear obliterated .
MRI
On MRI, uremic encephalopathy typically presents as bilateral T2/FLAIR hyperintensities involving the basal ganglia, thalamus, midbrain and mesial temporal lobes. Restricted diffusion may or may not be present to a varying degree, but is not characteristic of uremic encephalopathy. Enhancement is not a typical feature.
- lentiform fork sign: is a characteristic feature of uremic encephalopathy, in which the white matter surrounding the basal ganglia - the internal and external capsules and the medullary laminae - become hyperintense on T2/FLAIR
Treatment and prognosis
Dialysis is the primary treatment for uremic encephalopathy. This may be preceded by a period of peritoneal dialysis, which can be administered to ambulatory patients. Many patients ultimately require renal transplantation. Epileptic seizures, including non-convulsive seizures, occur in up to one-third of all uremic patients. In evaluating patients with seizures, it is essential to determine whether the seizure is the result of uremia or the consequence of some other coexisting or causative illness such as malignant hypertension with encephalopathy, intercurrent infection, dialysis disequilibrium syndrome, or cerebral infarction. Usually, the seizures caused by uncomplicated uremia are generalized, but focal motor seizures and epilepsia partialis continua occur .
Differential diagnosis
Other toxic encephalopathies such as acute liver failure and hepatic encephalopathy, metabolic disturbances such as disorders of glucose metabolism and disorders of water and electrolyte metabolism, and finally drug overdose and toxic exposures should also be considered.
The vast majority of toxic and metabolic disorders of the brain involve the deep gray nuclei (basal ganglia and thalamus) or the cerebral white matter. Typically, there is symmetric abnormality of the involved structures, which can provide a clue to the correct diagnosis .
Siehe auch:

 Assoziationen und Differentialdiagnosen zu urämische Enzephalopathie:
Assoziationen und Differentialdiagnosen zu urämische Enzephalopathie: