Codman-Tumor




























 nicht verwechseln mit: Codman-Dreieck
nicht verwechseln mit: Codman-DreieckChondroblastomas, also referred as Codman tumors, are rare benign cartilaginous neoplasms that characteristically arise in the epiphysis or apophysis of a long bone in young patients. Despite being rare, they are one of the most frequently encountered benign epiphyseal neoplasms in skeletally immature patients.
Epidemiology
Chondroblastomas represent less than 1% of all primary bone tumors, occurring predominantly in young patients (<20 years of age) with an overall male predilection .
Clinical presentation
Clinical presentation is non-specific and may include joint pain, muscle wasting, tenderness, and swelling/local mass. Chondroblastoma produces prostaglandins, which induce pain; moreover, due to its epiphyseal location, it can trigger synovial reaction .
Pathology
Malignant transformation has been seen in a small proportion of cases, with local and vascular invasion, and distant metastases.
Associations
Aneurysmal bone cysts can be seen secondarily to underlying chondroblastoma .
Histology
Microscopically they are composed of chondroblasts, chondroid matrix, cartilage with occasional giant multinucleated cells (which may lead to the incorrect diagnosis of giant cell tumor).
Calcium deposition surrounding the chondroblasts, which are typically polyhedral shape, results in typical "chicken-wire calcification" (pathognomonic) .
Location
Chondroblastomas most frequently arise in the epiphyses of long bones, with 70% occurring in the humerus (most frequent), femur and tibia . Approximately 10% are found in the hands and feet .
Radiographic features
Chondroblastomas are a prominent part of the differential diagnosis and mnemonics for radiolucent bone lesions and epiphyseal lesions .
Plain radiograph
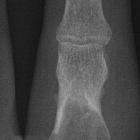
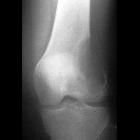
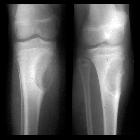
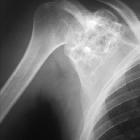
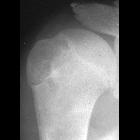
Chondroblastomas are seen as well defined lucent lesions, with either smooth or lobulated margins and a thin sclerotic rim, arising eccentrically in the epiphysis of long tubular bones (e.g., femur, humerus, or tibia) or apophyses such as the greater trochanter of the femur, greater tuberosity of the humerus, acromion, calcaneus, or talus. As they enlarge, they tend to extend to the metaphysis. Cortical breach is rare.
Internal calcifications can be seen in 40-60% of cases . They range in size from 1-10 cm, with most being 3-4 cm at diagnosis .
A joint effusion is seen in one-third of patients.
Among epiphyseal lesions, the presence of solid or layered periosteal reaction distant to the lesion (involving the diaphysis) is distinctive for chondroblastoma .
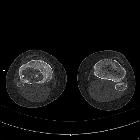
CT
CT demonstrates the plain film findings with better delineation of the relationship to the growth plate and articular surface. Solid periosteal reaction (seen in up to 50% of cases) and internal calcification (calcified matrix seen in ~50% of cases) and cortical breach are also more easily appreciated . Endosteal scalloping may be seen .
MRI
MRI is ideal for the evaluation of transphyseal or transcortical extension, and for demonstrating associated surrounding bone marrow and soft tissue edema, which is seen in a large proportion of cases .
These lesions have well-circumscribed, lobulated margins. Signal is variable :
- T1: intermediate signal
- T2: variable signal
- STIR: high signal
- T1 C+: heterogeneous moderate enhancement together with enhancement of surrounding bone and soft-tissue edema
Fluid-fluid levels may occasionally be seen (see fluid-fluid level containing bone lesions) presumably due to an associated aneurysmal bone cyst .
Treatment and prognosis
Treatment typically consists of curettage and packing of the resulting cavity with either bone or bone cement (polymethylmethacrylate). Radiofrequency ablation has also been used .
Unfortunately due to their proximity to the articular surface and growth plate complete eradication is difficult. As a result recurrence rates are relatively high (8-20%), and injury to the growth plate may result in growth arrest and limb-length discrepancy .
Treatment may be also complicated by growth plate injury, which can lead to growth arrest and limb length discrepancy.
Complications
Complications associated with chondroblastomas include pathological fractures and rarely, malignant transformation and pulmonary metastasis .
History and etymology
In 1931, this lesion was described by Ernest Armory Codman (1869-1940), American physician, as an epiphyseal chondromatous giant cell tumor of the proximal humerus, hence the term Codman tumor .
In 1942, Henry L Jaffe (1896-1979) and Louis Lichtenstein (1906-1977), American physicians and pathologists, designated this tumor as a benign chondroblastoma of bone .
Differential diagnosis
The differential is that of other lesions which have a predilection for the epiphysis or apophysis (see differential for an epiphyseal lesion). Specific lesions to be considered include :
- clear cell chondrosarcoma: see chondroblastoma vs clear cell chondrosarcoma
- osteomyelitis with abscess, e.g. Brodie abscess
- intraosseous ganglion
- giant cell tumor: older age group (closed physis)
Presence of bone marrow edema frequently seen surrounding chondroblastomas is helpful, as it is not a usual feature of chondromyxoid fibromas, giant cell tumors or enchondromas .

 Assoziationen und Differentialdiagnosen zu Chondroblastom:
Assoziationen und Differentialdiagnosen zu Chondroblastom: