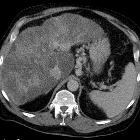
Fokale noduläre Hyperplasie














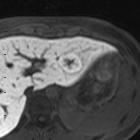












 nicht verwechseln mit: noduläre regenerative Hyperplasie
nicht verwechseln mit: noduläre regenerative HyperplasieFocal nodular hyperplasia (FNH) is a regenerative mass lesion of the liver and the second most common benign liver lesion (most common is a hemangioma). Many FNHs have characteristic radiographic features on multimodality imaging, but some lesions may be atypical in appearance. Focal nodular hyperplasias are typically asymptomatic lesions, usually requiring no treatment.
Epidemiology
Focal nodular hyperplasia is most frequently found in young to middle-aged adults, with a strong female predilection ; ~15% (range 10-20%) occur in men . Exogenous estrogens do not cause FNH, nor do they cause an increase in size of these masses. The isolated occurrence is the commonest but in up to 20% may be multiple and could occur with other lesions such as hemangiomas, etc.
Recent studies have shown that focal nodular hyperplasia can occur de novo after chemotherapy treatment with oxaliplatin (chemotherapy agent used for bowel and other types of cancer) .
Associations
Association with other benign lesions is commonly seen (~25%) :
- hepatic hemangiomas (most common)
- hereditary hemorrhagic telangiectasia
- arteriovenous malformations (AVM)
- anomalous venous drainage
- hepatic adenoma (possible but not proven)
- congenital absence of portal vein/portal vein atresia
- Budd-Chiari syndrome
- portal shunts
- idiopathic portal hypertension
- portal or pulmonary hypertension
Clinical presentation
These masses are either found incidentally on imaging or present due to mass effect, with right upper quadrant pain in 20% . Unlike hepatic adenomas, FNHs are only rarely complicated by spontaneous rupture and hemorrhage .
Pathology
The origin of focal nodular hyperplasia is thought to be due to a hyperplastic growth of normal hepatocytes with a malformed biliary drainage system, possibly in response to a pre-existent arteriovenous malformation . The arterial supply is derived from the hepatic artery whereas the venous drainage is into the hepatic veins. Focal nodular hyperplasia does not have a portal venous supply .
Focal nodular hyperplasia is divided into two types :
Typical FNH
Macroscopically, typical lesions demonstrate a mass which is often quite large with well-circumscribed margins but poorly encapsulated. A characteristic feature is a prominent central scar with radiating fibrous septa, but this is present in less than 50% of cases . A large central artery is usually present with spoke wheel like centrifugal flow (no portal veins).
Histologically the lesion is composed of abnormal nodular architecture, malformed vessels, and cholangiolar proliferation. Nearly normal hepatocytes are arranged in one to two cell-thick plates. Bile ductules are usually found at the interface between hepatocytes and fibrous regions . Kupffer cells are present .
There is no malignant potential .
Atypical FNH
An atypical FNH refers to a lesion which lacks the central scar and central artery, thus harder to distinguish from other lesions on gross inspection and imaging, or abnormal nodular architecture but with abnormal cholangiolar proliferation .
Atypical features also include pseudocapsule, lesion heterogeneity (more commonly seen in adenoma), non-enhancement of the central scar and intralesional fat .
Nodules can grow and disappear, and new nodules can appear even after resection .
Variants
Some authors also describe division of atypical focal nodular hyperplasia into several variants which include :
- telangiectatic variant: most common
- mixed hyperplastic and adenomatous variant
- lesions with large cell hepatocellular atypia
Radiographic features
As focal nodular hyperplasia is usually treated conservatively, accurate imaging is essential in preventing unnecessary intervention. Moreover, in women of childbearing age, hepatic adenoma is the chief differential diagnosis and biopsy of the latter can result in hemorrhage .
It should be noted that up to 20% of patients with a focal nodular hyperplasia will have multiple lesions and a further 23% will have hemangiomas .
Ultrasound
The echogenicity of both focal nodular hyperplasia and its scar is variable, and it may be difficult to detect on ultrasound. Some lesions are well-marginated and easily seen whereas others are isoechoic with surrounding liver. Detectable lesions characteristically will demonstrate a central scar with the displacement of peripheral vasculature on color Doppler examination. However, these findings are seen in only 20% of cases .
Contrast-enhanced ultrasound
- early arterial phase
- FNH will enhance relative to background liver
- typical FNA shows early arterial centrifugal filling
- prominent feeding vessel may be seen
- late arterial phase
- centrifugal filling (opposite to hemangioma and adenoma)
- portal venous phase
- sustained enhancement in the portal venous phase (as opposed to adenoma)
- unenhanced scar may be present
CT
A multiphase liver CT is ideal . On the non-contrast series, the lesion is usually hypo- or isoattenuating but may appear hyperattenuating if the rest of the liver is fatty. A hypoattenuating central scar can be seen in up to 60% of lesions >3 cm in size .
FNH demonstrates bright homogeneous arterial contrast enhancement except for the central scar which remains hypoattenuating . Enlarged central arteries may be seen.
In the portal venous phase, the lesion becomes hypo/isoattenuating to liver and poorly visualized in many studies. FNH is generally not associated with fat, calcification or hemorrhage.
The fibrotic scar demonstrates enhancement on delayed scans in up to 80% of cases .
MRI
Liver MRI is both sensitive (70%) and specific (98%).
- T1
- iso to moderately hypointense
- hypointense central scar
- T2
- iso to somewhat hyperintense
- hyperintense central scar
- contrast studies
- T1 C+ (Gd)
- intense early arterial phase enhancement, similar to CT
- isointense to liver on portal venous phase
- central fibrotic scar retains contrast on delayed scans
- T1 C+ (Eovist/Primovist)
- early arterial enhancement
- enhancement persists into delayed phases to a greater degree than the background liver due to the presence of normal hepatocytes and abnormal bile ductules
- fades toward background liver intensity on the delayed hepatobiliary phase, with a small amount of enhancement remaining (cf. adenomas, which are classically hypointense relative to liver on hepatobiliary phase)
- central fibrotic scar typically does not enhance on hepatobiliary phase
- T2* C+ (reticuloendothelial agent: SPIO)
- hypointense mass as a result of susceptibility signal loss due to uptake by Kupffer cells
- T1 C+ (Gd)
Nuclear medicine
Two radiopharmaceuticals can be used to image FNH – Tc-99m sulfur colloid and Tc-99m HIDA.
Sulfur colloid is taken up by the reticuloendothelial system, specifically the Kupffer cells which line the hepatic sinusoids . Because FNH is essentially focally malformed hepatic tissue, these lesions contain functioning Kupffer cells and take up technetium-99m sulfur colloid on nuclear imaging.
Classically, uptake pattern of Tc-99m sulfur colloid can be used to distinguish FNH from other liver lesions which do not contain normal liver cellularity, such as hepatic adenomas, HCC, and hepatic metastases. FNH is associated with normal or increased radiotracer uptake, whereas the others are associated with focal decreased uptake . Intense focal uptake is thought to be quite specific for FNH .
That being said, up to 30% of FNH lesions present with photopenia . Hepatic adenomas may rarely contain Kupffer cells and may be associated with normal uptake .
HIDA radiopharmaceuticals are taken up and excreted by hepatocytes in a similar way to bilirubin and bile. Since FNH contains all hepatic cell types (including hepatocytes and bile canaliculi) HIDA can be used to image FNH. Limited evidence suggests that HIDA scans might be more accurate than the more traditionally used sulfur colloid scan.
The usual HIDA scan findings consistent with an FNH lesion are increased blood flow, prompt hepatic uptake and delayed tracer clearance from the lesion. This pattern is reportedly seen in more than 90% of patients .
Treatment and prognosis
Focal nodular hyperplasia is benign, with no malignant potential and a minuscule risk of complication (rupture, hemorrhage) and thus are usually treated conservatively .
Differential diagnosis
General imaging differential considerations include:
- hepatic adenoma: usually more heterogeneous CT portal and delayed phases contrast washout; no gadoxetate retention on delayed phase MR and associated with fat, calcification or hemorrhage
- hepatocellular carcinoma (HCC): usually in cirrhosis; vascular invasion
- fibrolamellar (FL) HCC
- both FNH & FL-HCC commonly have a hypointense "central scar" representing fibrosis, so this feature is less useful for differentiation
- FL-HCC tends to appear more distinct from adjacent liver parenchyma on pre-contrast T1 / T2 weighted imaging
- often larger (>12 cm)
- calcification (uncommon in FNH), corresponding to necrosis and foreign body type reaction histopathologically
- 70% present with metastases, or evidence of biliary, vascular, and nodal invasion
- decreased activity on Tc-99m / sulfur colloid scan
- hypervascular hepatic metastases: usually multiple; CT portal and delayed phases hypodense (washout); older patients with known primary tumor
- hepatic hemangioma: peripheral and centripetal enhancement; blood vessels isodense; no central scar; only small ones with rapid enhancement simulate focal nodular hyperplasia (FNH)
- intrahepatic cholangiocarcinoma (hypoenhancing in earlier arterial/venous phases with delayed enhancement, dominant large central scar)
Practical points
- in the setting of cirrhotic liver, be wary of diagnosing a hypervascular lesion as an FNH unless you have definitely excluded HCC
- FNH typically has no capsule; if a hypervascular liver mass has a capsule, put HCC above FNH on the differential diagnosis
- there is likely overlap in appearance between FNH and inflammatory hepatic adenoma when using gadoxetic acid (Eovist/Primovist); if the patient has risk factors (e.g. metabolic syndrome), consider both in the differential diagnosis

 Assoziationen und Differentialdiagnosen zu Fokale noduläre Hyperplasie:
Assoziationen und Differentialdiagnosen zu Fokale noduläre Hyperplasie: