hepatocellular carcinoma






















































Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. It is strongly associated with cirrhosis, from both alcohol and viral etiologies. HCC constitutes approximately 5% of all cancers partly due to the high endemic rates of hepatitis B infection .
Epidemiology
Hepatocellular carcinoma is the fifth most common cancer in the world and is the third most common cause of cancer-related death (after lung and stomach cancer). The incidence of HCC is rising, largely attributed to a rise in hepatitis C infection .
The demographics are strongly influenced by the regions in which chronic hepatitis B infection is common, which account for over 80% of cases worldwide. The highest prevalence is in Asia.
In Western countries, the rate is lower and alcohol accounts for a greater proportion of cases.
Risk factors include :
- hepatitis B (HBV) infection: 10% 5-year cumulative risk
- hepatitis C (HCV) infection: 30% 5-year cumulative risk
- alcoholism: 8% 5-year cumulative risk
- biliary cholangitis: 5% 5-year cumulative risk
- food toxins, e.g. aflatoxins
- congenital biliary atresia
- inborn errors of metabolism
- hemochromatosis: ~20% 5-year cumulative risk
- alpha-1 antitrypsin deficiency
- type 1 glycogen storage disease
- Wilson disease
- tyrosinemia type I
- porphyrias
- obesity and diabetes mellitus
- chronic cholestatic syndromes
HCC is typically diagnosed in late middle age or elderly adults (average 65 years) and is more common in males (75% cases) . The tumor can also occur in the pediatric population; however, it is the second most common pediatric primary liver tumor after hepatoblastoma.
Fibrolamellar hepatocellular carcinoma is a distinct variant of HCC not associated with cirrhosis and has different demographics and risk factors.
Clinical presentation
The presentation is variable and, in affluent nations, is often found in the setting of screening programs for patients with known risk factors . Otherwise, presentation may include:
- constitutional symptoms
- jaundice
- portal hypertension from the invasion of the portal vein
- hepatomegaly/mass
- hemorrhage from tumor
Pathology
The origin of hepatocellular carcinomas is believed to be related to repeated cycles of necrosis and regeneration, irrespective of the cause. Also, the HBV and HCV genomes contain genetic material that may predispose cells to accumulate mutations or disrupts growth control, thus allowing for a second mechanism by which infection with these agents predisposes to HCC .
Macroscopic appearance
On gross pathology, hepatocellular carcinomas typically appear as pale masses within the liver and may be unifocal, multifocal or diffusely infiltrative at the time of presentation.
The macroscopic growth of HCCs is usually categorized into three subtypes: nodular, massive and infiltrative. Each has different radiological features, which are detailed below . The infiltrative subtype is characterized by a growth of multiple tiny nodules throughout the entire liver or an entire liver segment.
Microscopic appearance
Microscopically they range from well-differentiated to undifferentiated.
Markers
- alpha-fetoprotein (AFP) levels are elevated in 50-75% of cases
Radiographic features
Hepatocellular carcinomas can have a variety of appearances:
- massive (focal)
- large mass
- may have necrosis, fat and /or calcification
- nodular (multifocal)
- multiple masses of variable attenuation
- may also have central necrosis
- infiltrative (diffuse)
- may be difficult to distinguish from associated cirrhosis – they also have been called cirrhotomimetic-type HCC or cirrhosis-like HCC
Hepatocellular carcinoma receives most of its blood supply from branches of the hepatic artery, accounting for its characteristic enhancement pattern: early arterial enhancement with early "washout." Hence, small foci of HCC may be seen within a regenerative liver nodule as foci of arterial enhancement (nodule-in-nodule appearance) .
HCC uncommonly demonstrates a central scar similar to the FNH but may be differentiated by the absence of delayed contrast enhancement of the scar (as seen in FNH).
Rim enhancement on delayed post-contrast images causing a capsule-appearance is considered relatively specific for HCC (case 4).
Additionally, these tumors have the propensity to invade vascular structures, most commonly the portal vein, but also the hepatic veins, IVC, and right atrium. One should remember that a large number of patients will have concomitant cirrhosis, and thus also be at risk for bland portal vein thrombosis from synthetic dysfunction of clotting factors.
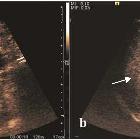
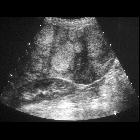
Ultrasound
Variable appearance depending on the individual lesion, size, and echogenicity of background liver. Typically:
- small focal HCC appears hypoechoic compared with normal liver
- larger lesions are heterogeneous due to fibrosis, fatty change, necrosis and calcification
- a peripheral halo of hypoechogenicity may be seen with focal fatty sparing (see the discussion below on the CT session)
- diffuse HCC may be difficult to identify or distinguish from background cirrhosis
- contrast-enhanced ultrasound
- arterial phase
- arterial enhancement from neovascularity
- portal venous phase
- decreased echogenicity relative to background liver ("wash out")
- tumor thrombus may be visible
- variants have been described with arterial phase hypovascularity with no enhancement or arterial enhancement with no "washout"
- arterial phase
CT
Several patterns can be seen, depending on the subtype of hepatocellular carcinoma. Enhancement pattern is the key to the correct assessment of HCCs.
Usually, the mass enhances vividly during late arterial (~35 seconds) and then washes out rapidly, becoming indistinct or hypoattenuating in the portal venous phase, compared to the rest of the liver.
Additionally, they may be associated with a wedge-shaped perfusion abnormality due to arterioportal shunts (APS), and this, in turn, can result in a focal fatty change in the normal liver or focal fatty sparing in the diffusely fatty liver . A halo of focal fatty sparing may also be seen around an HCC in an otherwise fatty liver .
Portal vein tumor thrombus can be distinguished from bland thrombus by demonstrating enhancement.
MRI
When seen in the setting of cirrhosis, small hepatocellular carcinomas need to be distinguished from regenerative and dysplastic nodules .
In general, MRI signal is:
- T1
- variable
- iso- or hypointense cf. surrounding liver
- hyperintensity may be due to
- intratumoral fat
- decreased intensity in the surrounding liver
- T1 C+ (Gd)
- enhancement is usually arterial ("hypervascularity")
- rapid "washout," becoming hypointense to the remainder of the liver (96% specific)
- this is because the supply to HCCs is predominantly from the hepatic artery rather than the portal vein
- rim enhancement may persist ("capsule")
- an imaging classification system (LI-RADS) has been developed to stratify lesions
- T1 C+ (Eovist/Primovist)
- similar to assessment with extracellular Gd, but evaluation of the hepatobiliary phase requires care
- arterial hyperenhancement with washout assessed on the portal venous phase
- washout on transitional phase (3 minutes delayed) is less reliable (see: Eovist and LI-RADS)
- similar to assessment with extracellular Gd, but evaluation of the hepatobiliary phase requires care
- T2: variable, typically moderately hyperintense
- C+ post-SPIO (iron oxide): increases sensitivity in diagnosing small HCCs
- DWI: intratumoral high signal; increases sensitivity and specificity
DSA: angiography
- hypervascular tumor
- threads and streaks pattern: sign of tumor thrombus in the portal vein
Staging and classification
The typical TNM staging system seen in most other epithelial cancers is not as prognostically useful for stratification of patients with hepatic cancers.
There are several substitute staging systems used in guiding therapy for hepatocellular carcinoma . An imaging classification system (LI-RADS) has been developed to stratify lesions in an at-risk liver.
Treatment and prognosis
If the lesion is small then resection is possible (partial hepatectomy) and may result in the cure. The remarkable ability of the liver to regenerate means that up to two thirds of the liver can be resected .
Liver transplantation is also a curative option. To be suitable for liver transplantation it is agreed that certain criteria should be met (see Milan criteria).
If neither of these options is possible, then a variety of options exist including chemotherapy, transarterial chemoembolisation (TACE), thermal ablation (RFA, cryoablation, or microwave ablation) and selective internal radiation therapy (SIRT) .
If a tumor is resectable, then 5-year survival is ~45% (range 37-56%) .
Metastasis occurs in the final stages of disease (IVa) and carries a poor prognosis . The most frequently involved sites are the lung, adrenal glands, lymph nodes, and bone.
Differential diagnosis
General imaging differential considerations include:
- hypervascular hepatic metastases
- metastases to a cirrhotic liver are rare, often due to primary endocrine tumor
- less venous invasion
- focal nodular hyperplasia (FNH)
- no vascular invasion or neovascularization
- may have non-enhancement "halo" around mass or in central scar
- early arterial Eovist enhancement persists into delayed phases
- Tc-99m sulphur colloid 80% positive
- hepatic adenoma
- different demographics and risk factors
- intrahepatic cholangiocarcinoma
- peripheral location
- biliary obstruction
- delayed enhancement
- THADs
- primary hepatic lymphoma
- hepatic tuberculoma

 Assoziationen und Differentialdiagnosen zu hepatozelluläres Karzinom:
Assoziationen und Differentialdiagnosen zu hepatozelluläres Karzinom: