Hypoxic-ischemic encephalopathy (adults and children)





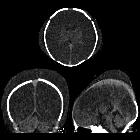










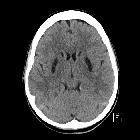




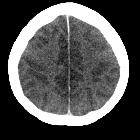























Hypoxic-ischemic encephalopathy in adults and older children (i.e. not neonates), also known as global hypoxic-ischemic injury, is seen in many settings and often has devastating neurological sequelae.
For a discussion of neonatal hypoxia, refer to neonatal hypoxic-ischemic encephalopathy.
Epidemiology
Hypoxic-ischemic cerebral injury occurs at any age, although the etiology is significantly different:
- older children: drowning and asphyxiation remain common causes
- adults: more often a result of cardiac arrest or cerebrovascular disease, with secondary hypoxemia/hypoperfusion
Clinical presentation
Patients typically present to hospital following an acute event (near-drowning, asphyxia, cardiac/respiratory arrest). They are usually intubated and have a history of prolonged resuscitation.
Pathology
Severe global hypoxic-ischemic injury in this population primarily affects the grey matter structures:
- basal ganglia
- thalami
- cerebral cortex (in particular the sensorimotor and visual cortices, although involvement is often diffuse)
- cerebellum
- hippocampi
The predominance of grey matter injury is due to its high metabolic requirement for oxygen and glucose to supply a large number of synapses. This makes grey matter more susceptible to hypoxic-ischemic injury . It also contains most of the dendrites where postsynaptic glutamate receptors are located. They are therefore the sites most susceptible to the effects of glutamate excitotoxicity (i.e., damage to nerve cells by excessive stimulation by glutamate).
Neurologic injury is caused by hypoxia (secondary to carbon monoxide toxicity, near drowning, etc.) or interruption of blood flow (usually from cardiac arrest or hanging). There are often secondary effects of hypoxia on cardiac myocytes, causing reduced cardiac output and causing further secondary neurological injury. Hypoxia alone rarely causes significant brain damage unless it is profound and prolonged.
Although cerebellar injury can be seen in neonates, it tends to be more common in older patients. The reason for this predilection is not entirely clear, but it has been suggested that the relative immaturity of Purkinje cells (which are normally exquisitely sensitive to ischemic damage) in neonates somehow protects the cerebellar cortex .
Radiographic features
CT
- diffuse edema with effacement of the CSF-containing spaces
- decreased cortical grey matter attenuation with a loss of normal grey-white differentiation
- decreased bilateral basal ganglia attenuation
- reversal sign: reversal of the normal CT attenuation of grey and white matter, demonstrated within the first 24 hours in a small number of these patients
- it has been proposed that this finding is due to the distension of deep medullary veins secondary to partial obstruction of venous outflow from the elevated intracranial pressure caused by diffuse edema
- the result is that the cerebral white matter is of higher attenuation than the cortical grey matter
- white cerebellum sign: has been described in at least one study as a component of the reversal sign and in which there is diffuse edema and hypoattenuation of the cerebral hemispheres with sparing of the cerebellum and brainstem, resulting in apparent high attenuation of the cerebellum and brainstem relative to the cerebral hemispheres
- linear hyperdensity outlining the cortex as well as linear cortical enhancement (later and less evident signs) correspond to cortical laminar necrosis
- pseudosubarachnoid hemorrhage: cerebral edema and a resultant decrease in parenchymal attenuation and swelling and engorgement and dilatation of the superficial venous structures due to an increased intracranial pressure result in the subarachnoid space appearing filled with blood that appears hyperdense.
Both the reversal sign and the white cerebellum sign indicate severe injury and a poor neurologic outcome .
MRI
Diffusion-weighted MR imaging is the earliest imaging modality to become positive, usually within the first few hours after a hypoxic-ischemic event due to early cytotoxic edema. During the first 24 hours, there may be restricted diffusion in the cerebellar hemispheres, basal ganglia, or cerebral cortex (in particular, the perirolandic and occipital cortices) . The thalami, brainstem or hippocampi may also be involved. Diffusion-weighted imaging abnormalities usually pseudo-normalize by the end of the 1week .
As in younger patients, conventional T1 and T2 weighted images are often normal or demonstrate only very subtle abnormalities. In the early subacute period (24 hours to 2 weeks), conventional T2 weighted images typically become positive and show increased signal intensity and swelling of the injured grey matter structures .
T1 hyperintensities indicating cortical laminar necrosis become evident after two weeks. This hyperintense signal does not represent hemorrhage, and it is believed to be caused by the accumulation of denatured proteins in dying cells. This hyperintensity can also be seen within a few days on FLAIR .
Differential diagnosis
- molybdenum cofactor deficiency: very rare lethal genetic disease
See also
Siehe auch:
- hypoxischer Hirnschaden
- kortikale laminäre Nekrose
- Pseudosubarachnoidalblutung
- reversal sign
- Kohlenmonoxidintoxikation
- neonatal hypoxic-ischaemic encephalopathy
- hypoxic-ischaemic injury in older children and adults
- global cerebral ischemia
- Globus pallidum hypodens
und weiter:
- Ischämischer Schlaganfall
- intrakranielle Thrombektomie
- dense cerebellum sign
- Enzephalopathie
- fetal hypoxia
- pseudosubarachnoid sign
- MR spectroscopy of hypoxic-ischemic injury
- CT in hanging
- hypoxic brain damage T2
- Hirntoddiagnostik
- hypoxic brain injury with pseudosubarachnoid haemorrhage
- hypoxic brain damage MRI
- neonatale hypoxisch-ischäme Enzephalopathie
- hypodense Basalganglien

 Assoziationen und Differentialdiagnosen zu hypoxic-ischemic brain injury:
Assoziationen und Differentialdiagnosen zu hypoxic-ischemic brain injury:

