Leigh-Syndrom








Leigh syndrome, also known as subacute necrotizing encephalomyelopathy (SNEM), is a mitochondrial disorder with progressive neurodegeneration that invariably leads to death, usually in childhood.
Epidemiology
Leigh syndrome is encountered in approximately 1 in 40,000 births, although some populations have much higher incidence (e.g. in Quebec, Canada) . There is no known gender or racial predilection .
Clinical presentation
Typically, symptoms become evident before the age of 2, with the presentation in later childhood (juvenile form) or adulthood (adult form) being uncommon. Symptoms include :
- psychomotor delay/regression
- superimposed signs of basal ganglia and brainstem dysfunction
- ataxia
- ophthalmoplegia
- dystonia
- respiratory rhythm disturbance
- cranial nerve palsies
Pathology
Leigh disease is one of many mitochondrial disorders, due to a broad range of genetic mutations in both nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) .
Nuclear DNA mutations are more common (~75%) and are inherited in a Mendelian fashion with both autosomal recessive and X-linked inheritance encountered .
Cases due to mitochondrial DNA are less common (25%) and only inherited from the mother .
Some mutations (e.g. SURF1) are particularly devastating .
Chronic energy deprivation leads to histological features such as :
- spongiform degeneration
- capillary proliferation
- demyelination
- neuronal loss
- gliosis
These findings are similar to those seen in infarction .
Genetics
The inheritance pattern may be either autosomal recessive or X-linked.
Markers
CSF lactate may be elevated.
Radiographic features
CT
CT demonstrates regions of low-density matching areas of the abnormal T2 signal on MRI (see below) . Occasionally some of these areas can show contrast enhancement .
MRI
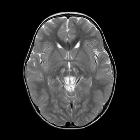
MRI abnormalities are heterogeneous and differ depending on the underlying genetic abnormality . Generally, the distribution tends to be symmetrical.
- T2: characterized by high signal typically in :
- brainstem
- periaqueductal grey matter
- medulla
- midbrain
- putamen: characteristic but not always present
- other sites of T2 signal change include:
- the remainder of the corpus striatum [globus pallidus and caudate nucleus (heads)]
- subthalamic nuclei
- substantia nigra
- thalami
- involvement of cerebral or cerebellar white matter is unusual
- T1: usually demonstrates reduced signal in T2 abnormal areas, although some areas of hyperintensity can be seen, as can some enhancement
- DWI: in the acute setting some restricted diffusion may be evident
- MR spectroscopy
- elevated choline
- occasionally elevated lactate
- reduced NAA
Treatment and prognosis
Prognosis is poor, with death usually occurring in childhood. The later the onset, the slower the deterioration. Death is most frequently due to respiratory failure .
The factors associated with a worse outcome are :
- disease onset before 6 months of age
- admission to an intensive care
- brainstem lesions
- MRS lactate peak
History and etymology
It is named after Archibald Denis Leigh, British neuropathologist, who first described the condition in 1951 .
Differential diagnosis
- Wernicke encephalopathy (WE)
- similar appearance but different demographics
- mammillary bodies not involved in Leigh disease
- enhancement more common in WE
- hemorrhagic change more common in WE
- other mitochondrial disorders
- brainstem and basal ganglia involvement less pronounced
- acute necrotizing encephalitis of childhood
- lactate levels are usually normal
- Biotin-thiamine-responsive basal ganglia disease
Siehe auch:
und weiter:
- T2 hyperintense Basalganglien
- dysmyelinating disorders
- Leukodystrophie
- choline:creatine ratio
- Kohlenmonoxidintoxikation
- Chorea Huntington
- Kearns-Sayre-Syndrom
- Mitochondriale Encephalomyopathie mit Lactatacidose und Schlaganfall-ähnlichen Episoden (MELAS)
- mitochondrial encephalopathy with lactic acidosis and stroke-like episodes
- Pyruvat-Dehydrogenase-Mangel
- Pyruvatdehydrogenase
- Neonatale Leukodystrophie
- putaminal necrosis

 Assoziationen und Differentialdiagnosen zu Leigh-Syndrom:
Assoziationen und Differentialdiagnosen zu Leigh-Syndrom:

