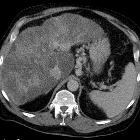
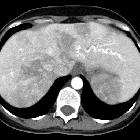
pulmonary arteriovenous malformation








































Pulmonary arteriovenous malformations (PAVMs) are rare vascular anomalies of the lung, in which abnormally dilated vessels provide a right-to-left shunt between the pulmonary artery and vein. They are generally considered direct high flow, low-resistance fistulous connections between the pulmonary arteries and veins.
Epidemiology
There is a recognized female predilection with F:M ratios ranging around 1.5 to 1.8:1. The estimated incidence is thought to be around 2-3 per 100,000 .
Clinical presentation
Despite most patients being asymptomatic, the connection between the venous and arterial system can lead to dyspnea (due to right-to-left shunting), as well as embolic events (due to paradoxical emboli). Although it is assumed that the vascular defects are present at birth, they are seldom manifested clinically until adult life when the vessels have been subjected to pressure over several decades. Clinically a murmur or bruit may be audible over the lesion (especially if peripheral). There are be an extremely variable age of presentation from infant to old age although most patient within the first three decades of life.
Pathology
In congenital cases, they are considered to result from a defect in the terminal capillary loops which causes dilatation and the formation of thin-walled vascular sacs. They can be multiple in around one-third of cases.
Classification
They can be classified as simple, complex or diffuse .
- simple type: commonest; has a single segmental artery feeding the malformation; the feeding segmental artery may have multiple subsegmental branches that feed the malformation but must have only one single segmental level
- complex type: have multiple segmental feeding arteries (~20% )
- diffuse type: rare (~5% of lesions); the diffuse form of the disease is characterized by hundreds of malformations; some patients can have a combination of simple and complex AVMs within a diffuse lesion
Another older embryological based classification proposed by Anatwabi et al. in 1965 is like
- group I: multiple small arteriovenous fistulas without an aneurysm
- group II: large arteriovenous aneurysm
- group III
- large arteriovenous aneurysm (central)
- large arteriovenous aneurysm with anomalous venous drainage
- multiple small arteriovenous fistulae with anomalous venous drainage
- group IV
- large venous aneurysm with systemic arterial communication
- large venous aneurysm without fistula
- group V: anomalous venous drainage with fistulae
Location
These are often unilateral. Although can potentially affect any part of the lung, there is a recognized predilection towards the lower lobes (50-70%) .
Associations
PAVMs have been described in association with a number of conditions.
- hereditary hemorrhagic telangiectasia (HHT) frequently have PAVMs ; it is reported that at least 33% of those with a single PAVM and at least 50% of those with multiple PAVM's have HHT
In addition, PAVMs have been found in:
- hepatic cirrhosis (as part of the hepatopulmonary syndrome)
- schistosomiasis
- mitral stenosis
- trauma
- previous cardiac surgery (e.g. Glenn and Fontan procedures for cyanotic congenital heart disease)
- actinomycosis: thoracic actinomycosis infection
- Fanconi syndrome
- metastatic thyroid carcinoma
- tuberculosis (Rasmussen aneurysm)
Radiographic features
A number of modalities are available for the diagnosis of PAVMs, including contrast echocardiography, radionuclide perfusion lung scanning, computed tomography (CT), magnetic resonance imaging (MRI), and, the gold standard, pulmonary angiography .
Plain radiograph
Pulmonary varix (dilated vessel) may be apparent as a non-specific soft tissue mass, often with a relatively unusual orientation compared to adjacent vessels. More than one raises the possibility of hereditary hemorrhagic telangiectasia .
CT
CT is often the diagnostic imaging modality of choice. The characteristic presentation of a PAVM on non-contrast CT is a homogeneous, well-circumscribed, non-calcified nodule up to several centimeters in diameter or the presence of a serpiginous mass connected with blood vessels . Occasionally associated phleboliths may be seen as calcifications. Contrast injection demonstrates enhancement of the feeding artery, the aneurysmal part, and the draining vein on early-phase sequences.
MRI
Three-dimensional contrast-enhanced MR angiography is considered the MR technique of choice for imaging vascular structures in the thorax . Most lesions within the lung have relatively long relaxation time and produce medium to high-intensity signals. Lesions with rapid blood flow within resulting in a signal void and produce low-intensity signals.
Treatment and prognosis
Treatment options include:
- trans-catheter coil embolization
- surgery (historically treated with surgery)
Once successfully treated (embolotherapy, surgical resection), the prognosis is generally good for an individual lesion.
Complications
- cyanosis (due to the right to left shunt)
- high output cardiac failure
- polycythemia
- paradoxical cerebral embolism
History and etymology
The first description of pulmonary arteriovenous malformation was reported by T Churton in 1897.
Differential diagnosis
Possible imaging differential considerations include:
- abnormal systemic vessels
- highly vascular parenchymal mass
- other congenital or acquired pulmonary arterial or venous lesions (e.g. pulmonary varix)
- retroperitoneal varices
- bronchoceles: on contrast scans
Siehe auch:
- Tuberkulose
- Leberzirrhose
- Arteriovenöse Malformation
- Morbus Osler-Weber-Rendu
- solitärer pulmonaler Rundherd
- variköse Erweiterung der Pulmonalvenen
- Mitralklappenstenose
- thorakale Aktinomykose
- Rasmussen-Aneurysma
- Hepatopulmonales Syndrom
- Bronchozele
- meandernde Pulmonalvenen
- pulmonary arteriovenous fistula in hereditary hemorrhagic telangiectasia
- pulmonary arteriovenous malformation in Rendu-Osler-Weber disease
- kindliche Lungenläsionen
- hyperdense Mukusimpaktion
- Varianten Pulmonalarterie
und weiter:

 Assoziationen und Differentialdiagnosen zu arteriovenöse Malformationen der Lunge:
Assoziationen und Differentialdiagnosen zu arteriovenöse Malformationen der Lunge: