Spontaneous craniospinal hypotension
























Intracranial hypotension, also known as craniospinal hypotension is defined as cerebrospinal fluid (CSF) pressure <6 cm H2O in patients with clinical presentation compatible with intracranial hypotension, namely, postural headache, nausea, vomiting, neck pain, visual and hearing disturbances, and vertigo . It most commonly results from a CSF leak somewhere along the neuraxis.
Intracranial hypotension can broadly be divided into:
Epidemiology
Spontaneous intracranial hypotension is typically encountered in middle age (30-50 years of age) and has a predilection for women (F:M, 2:1). Of interest, this is a similar demographic to pseudotumor cerebri, which is believed to be an unrecognised predisposing factor.
Epidemiology of secondary intracranial hypotension is variable and matches that of the underlying cause.
Clinical presentation
The condition often presents as a positional headache which is relieved by lying in a recumbent position, usually within 15-30 minutes .
In cases of traumatic or iatrogenic intracranial hypotension, a history of abundant, clear rhinorrhea or otorrhea can sometimes be elicited. Such would be the case with cerebrospinal fluid (CSF) leak following sinus surgery, pituitary surgery or with basal skull fractures.
It is confirmed by an opening pressure on lumbar puncture of less than <6 cm H2O . Note that if done fluoroscopically, lumbar puncture should be performed in a lateral position to allow for accurate measurement of pressure.
Occasionally, presentation is more sinister, with reported cases of decreased level of consciousness and coma .
Pathology
Intracranial hypotension most commonly results from a CSF leak somewhere along the neuraxis, and leads to alterations in the equilibrium between the volumes of intracranial blood, CSF, and brain tissue (Monro-Kellie hypothesis). A decrease in CSF volume leads to compensatory dilatation of the vascular spaces, mostly the venous side due to its higher compliance.
Spontaneous intracranial hypotension (SIH) usually is the result of a CSF leak in the spine. Causes include :
- spontaneous dural dehiscence of meningeal diverticula (perineural cyst)
- secondary to degenerative dural tears (typically related to calcified thoracic disc protrusions)
- congenital focal absence of dura (nude nerve root) - rare
- CSF-venous fistula - rare
Much less frequently, but increasingly recognized, SIH can result from small medial sphenoid meningoceles, often in patients with undiagnosed pseudotumor cerebri. It is also more commonly seen in connective tissue disorders, including Marfan syndrome, Ehlers-Danlos syndrome (type II), and autosomal dominant polycystic kidney disease (ADPKD). In many instances, the manifestation of these disorders may be subtle or not fit a defined syndrome .
CSF leaks can also be iatrogenic, following lumbar puncture, surgery, secondary to over-shunting, or trauma.
Radiographic features
Imaging is crucial both for confirming the diagnosis of intracranial hypotension and identifying the location of the leak. The latter is discussed below in the Imaging Strategy section.
CT
Described features of intracranial hypotension include:
MRI
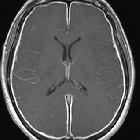
The most common qualitative finding is pachymeningeal thickening and enhancement followed by dural venous engorgement, tonsillar herniation, and subdural collection; however, these features are not always present, which is why quantitative findings are very helpful in making a more accurate diagnosis on MRI.
- qualitative signs
- pachymeningeal enhancement (most common finding): becomes less prevalent over time after the onset of symptoms; hence, in patients with a chronic duration of symptoms in whom clinically the headache pattern also changes from orthostatic to atypical constant headache, the absence of dural enhancement may hinder the diagnosis of SIH
- increased venous blood volume
- venous distension sign: rounding of the cross-section of the dural venous sinuses
- prominence of inferior intercavernous sinuses is not a sensitive or specific finding; however, it is important to recognize that in cases of SIH, should not be mistaken for a pituitary lesion
- intracranial venous thrombosis is a well-recognized, albeit uncommon, complication and may involve the cortical veins and/or dural venous sinuses
- enlargement of the pituitary gland
- subdural effusions and eventual subdural hematomas
- diffuse cerebral edema
- reduced CSF volume
- sagging brainstem and acquired tonsillar ectopia
- droopy penis sign: perhaps only an Australianism to denote the shape of the downward drooping splenium of the corpus callosum
- decreased fluid within the optic nerve sheath
- quantitative signs
- mamillopontine distance <5.5 mm
- pontomesencephalic angle <50˚
- interpeduncular angle <40.5° measured in the axial plane at or immediately below the level of the mammillary bodies
A useful mnemonic to remember the aforementioned features can be found here.
Imaging strategy
Identifying the site of CSF leakage can be challenging, especially in spontaneous cases, and embarking upon imaging can be daunting, as the leak can be located anywhere along the neuraxis and leaks can vary dramatically in their rate ranging from large volume rapid leaks to slow leaks to intermittent/inapparent leaks. As a result, no single examination is guaranteed to confirm and localize the abnormality.
Assuming the site is not likely to be cranial, a suggested imaging strategy is as follows :
- fast high volume leak: digital subtraction myelography or ultrafast/dynamic CT myelography
- no leak (?intermittent): MRI myelography (with intrathecal Gadolinium) or nuclear medicine myelography
1. MRI of the brain with contrast
MRI of the brain is an essential first step, needed to confirm the diagnosis of intracranial hypotension (see above) and rule out other unexpected pathology. In the setting of prior skull fracture or surgery, the addition of fat-saturated thin T2 weighted sequences are helpful in identifying an intracranial dural defect. This should also be added presumptively in patients who may have had undiagnosed pseudotumor cerebri (overweight young females) and targeted to the middle cranial fossa.
2. Speculative blood patch
If the diagnosis is beyond a doubt, with classic symptoms and imaging findings, and no likely localizing history is present (i.e. no trauma, surgery, history of or suspicion of antecedent pseudotumor cerebri), a speculative non-targeted lumbar epidural blood patch is a reasonable next step without necessarily trying to identify/confirm the location of leak .
In many cases, non-targeted lumbar epidural blood patch will not result in a permanent cure, and further imaging is required.
3. CT myelography or MRI spine
If confirmation of a leak is required (i.e. intracranial imaging and/or symptoms not definitive), and/or localization, further imaging is necessary.
CT myelography is useful in identifying the location of CSF leak and presence of calcified disc protrusions or osteophytes.
MRI of the spine is also useful, provided it is performed with fat-saturated T2 imaging allowing for the visualization of accumulation of CSF in the epidural space. On T1 imaging reduced signal in the epidural space may also be seen but is subtle.
It should be noted that if the amount of leaked CSF is large, the distribution of fluid should not be interpreted as necessarily representing the site of leak . CSF in the epidural space can migrate over significant distances and pool depending on patient position and anatomy.
A particular example of this is pooling of contrast / CSF at the C1/2 level posteriorly. This can be incorrectly attributed to a local leak, when in fact this is not the case. This is referred to as a false localizing sign .
4a. Fast leaks
CT myelography can be repeated, with the lumbar puncture performed on the CT table using CT fluoroscopy.
Digital subtraction myelography has the best temporal resolution, and with newer equipment allowing Dyna-CT, can also obtain cross-sectional images.
4b. No or intermittent leaks
MR myelography (with intrathecal gadolinium) is not approved by FDA and is associated with neurotoxicity, particularly if renal function or CSF hydrodynamics are compromised but it has been tried in some cases when other methods have failed to demonstrate the source of CSF leak . MR myelography with intrathecal gadolinium has been shown to be more sensitive than CT in that respect .
Nuclear medicine myelography can also be employed, using intrathecal Indium-DTPA, with images obtained typically at 1, 2, 4, 24 and in some instances even 48 hours post-injection . Localization can be seen as a focal accumulation of activity. In some cases, only indirect evidence of a leak being present 'somewhere' is available, which is only really useful in instances where the diagnosis of CSF leak remains uncertain. Indirect evidence includes :
- early accumulation of activity within the urinary tract (kidneys/bladder) at 4 hours
- absent activity over the cerebral convexities at 24 hours
- rapid loss of activity from within the CSF space
Cranial leak location
The presence of CSF rhinorrhea or CSF otorrhea or history of skull base surgery or trauma is a localizing sign and in such instances, targeted imaging of the base of the skull should be performed.
In most instances, an MRI (see above) and CT of the base of skull with thin bone algorithm images is sufficient for identifying bony defects in the skull base, including acute or long-standing fractures, congenital bony defects (e.g. meningoceles) or other focal deficiencies.
Treatment and prognosis
A non-targeted epidural blood patch is often used in cases of spontaneous intracranial hypotension, on the assumption that the leak is from the spine, with variable success . When successful, headaches resolve within 72 hours of intervention . Subdural effusions can resolve within a few days to weeks. Larger subdural collections often require far longer to resolve .
In cases where such speculative treatment fails, localization of the CSF leak is required, allowing for targeted epidural blood patch or surgical intervention .
Targeted epidural blood patch can either be performed using a translaminar approach, as for initial non-targeted injections or aimed at entering the ventral epidural space using a transforaminal approach at one or more levels and potentially from both sides .

 Assoziationen und Differentialdiagnosen zu Liquorunterdrucksyndrom:
Assoziationen und Differentialdiagnosen zu Liquorunterdrucksyndrom: