gastrointestinal stromal tumor






























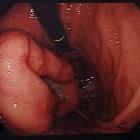

























Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. They account for ~5% of all sarcomas, and are mostly found within stomach and mid-distal small bowel. They respond remarkably well to chemotherapy.
Terminology
Previously these tumors have been variably referred to as leiomyomas, leiomyosarcomas, and leiomyoblastomas, as they were difficult to differentiate on histopathology until a characteristic expression of c-KIT and CD34 antigens was identified.
Epidemiology
- incidence
- overall uncommon when compared to gastrointestinal carcinoma, estimated ~0.0015%
- small autopsy reports suggest the incidence of tiny subclinical tumors ("tumorlets") may be as high as 23% in those over 50 years old
- age
- usually occur after 40 years of age, most seen in older patients
- may present earlier and often multiple in tumor syndromes (see below)
- gender
- possible slight male predilection
- by location
- esophagus: uncommon (versus leiomyomas)
- stomach: most common site, most common gastric sarcoma
- small bowel: second most common site
- colon and rectum: most common rectal sarcoma
Associations
The vast majority of GISTs are sporadic; however, they are recognized to occur in the following syndromes :
Clinical presentation
Clinical presentation is variable and reflects the variability of appearance, location, and biological behavior.
- small tumors are more likely to be asymptomatic, regardless of location
- tend to grow intramural, bulging intraluminal or extramural if large enough
- uncommon to cause dysphagia or bowel obstruction until very large
- larger tumors are known to ulcerate and cause gastrointestinal hemorrhage
- may present with non-specific gastrointestinal symptoms (e.g. abdominal pain, nausea, vomiting)
- aggressive tumors may present with metastases or symptoms relating to local disease
Pathology
GISTs are typically submucosal tumors, and the overlying mucosa often remains intact on pathological and imaging assessment.
They are believed to arise from the interstitial cells of Cajal , with 95% staining positive for CD117 (c-KIT) and 70% for CD34 . The former is a tyrosine kinase growth factor receptor and the target of ST-571 (imatinib/Gleevec/Glivec) .
Grading GISTs requires an assessment of both tumor size and mitotic index . Smaller lesions have less aggressive biological behavior, as do stomach GISTs when compared to tumors elsewhere along the gastrointestinal tract .
Macroscopic appearance
They are rounded with frequent hemorrhage. Larger tumors may also demonstrate necrosis and cystic change . Size is variable, ranging from 1 to 30 cm .
Histology
Histology demonstrates a relatively cellular tumor composed of spindle cells (70-80%) and plump epithelioid cells (20-30%) . They appear to arise from the muscularis propria layer.
Location and classification
GISTs occur anywhere along the gastrointestinal tract. Common sites of involvement include :
- stomach: 70% (most common gastric sarcoma)
- small intestine: 20-25%
- anorectum: 7% (most common rectal sarcoma)
- more likely to be high grade
- colon
- esophagus (uncommon)
In addition, extra-gastrointestinal GISTs are known to occur in the mesentery, omentum, and retroperitoneum . Metastatic lesions may also be seen in cases of malignant extra-gastrointestinal GISTs .
Radiographic features
Specific appearances will vary according to location (see above), but in general, these tumors appear as rounded soft tissue masses, arising from the wall of a hollow viscus (most commonly the stomach) with an endoluminal or exophytic growth. Mucosal ulceration is present in 50% of cases with large necrotic cavities communicating with the lumen also seen.
Plain radiograph
When large, secondary signs of the tumor may be visualized by radiograph, e.g. soft tissue density displacing bowel loops.
Fluoroscopy
On upper abdominal studies, filling defect projecting from the wall of the stomach may be seen, with overlying ulceration or cavitation. The tumor margins are normally seen as smooth and may form right or obtuse angles with the adjacent mucosa due to its intramural origin.
CT
Appearances vary with size and location. Typically the mass is of soft tissue density with central areas of lower density when necrosis is present (usually in larger tumors) that occasionally appear as fluid-fluid levels.
As the tumors are often exophytic, it can be difficult to delineate them on CT if the stomach is distended with barium, though the non-enhancing central necrotic area may be helpful. Deep crescent-shaped ulceration demonstrating an internal air-fluid level may be referred to as the Torricelli-Bernoulli sign .
Enhancement is typically peripheral (due to central necrosis) . Calcification is uncommon (3%) .
Metastases (distant, peritoneal, omental) or direct invasion into adjacent organs may be seen in more aggressive lesions. Lymph node enlargement is not a feature .
The diagnosis of malignant GIST requires histopathologic analysis, but certain characteristics suggest malignancy, which develops in 10-30% of these lesions. These include exogastric growth, diameter >5 cm, central necrosis, and extension to other organs.
MRI
The presence of necrosis, hemorrhagic and cystic change make appearances variable:
- T1
- low signal intensity solid component
- enhancement is usually present, and predominantly peripheral in larger lesions
- T2: high signal intensity solid component
Nuclear medicine
GISTs are FDG-avid tumors and F-18 FDG PET-CT can be used for initial staging and treatment response assessment . Areas of central necrosis can be photopenic on PET-CT (i.e. have low or absent tracer uptake) .
Treatment and prognosis
The Choi response criteria are used to assess treatment .
Surgical en-bloc resection is the primary mode of therapy for GISTs . Up to 50% of all GISTs will have evidence of metastatic disease at the time of presentation , which significantly impacts prognosis.
Adjuvant chemotherapy with ST-571 (imatinib) is effective in the majority of cases and has had a dramatic impact on prognosis even with only one year of therapy, reducing recurrence at one year from 17% to 3% . Longer treatment regimes (2-3 years of imatinib) are currently under investigation . Other second-line agents (e.g. sunitinib) are also being studied and used for patients with imatinib-resistant tumors.
Historically, before ST-571 (imatinib) up to 85% of tumors would locally recur or develop subsequent distal metastases despite treatment and had proven to be resistant to standard chemotherapy .
Fluorine-18 FDG PET-CT may be used in monitoring treatment response and is considered superior to CT in monitoring treatment response in the initial phases of treatment .
Differential diagnosis
General imaging differential considerations include:
- gastrointestinal leiomyoma
- most common in the esophagus, accounting for 75% of mesenchymal tumors
- rare in the remainder of the GI tract
- gastrointestinal leiomyosarcoma: rare
- gastrointestinal lymphoma / gastric lymphoma
- lymphadenopathy uncommon for GIST
- more extensive mural thickening
- there is often associated aneurysmal dilatation
- gastrointestinal schwannoma
- typically homogeneous attenuation
- tend to lack cystic change
- gastrointestinal carcinoid
- more common in the small bowel
- polypoid/plaque-like growth
- hyperenhancing
- mesenteric metastases with stranding (characteristic “spoke-wheel” appearance)

 Assoziationen und Differentialdiagnosen zu Gastrointestinaler Stromatumor:
Assoziationen und Differentialdiagnosen zu Gastrointestinaler Stromatumor:






