ependymomas




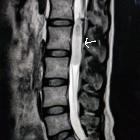









Ependymomas represent a relatively broad group of glial tumors most often arising from the lining the ventricles of the brain or the central canal of the spinal cord. They account for ~5% of all neuroepithelial neoplasms, ~10% of all pediatric brain tumors and up to 33% of brain tumors occurring in those less than 3 years of age.
Ependymomas can occur anywhere within the neuraxis, but distribution is not even, with the posterior fossa being most common, followed by supratentorium and lastly spinal cord. This distribution correlates with molecularly distinct tumors which in turn have different epidemiology and prognosis :
- posterior fossa: 60%
- molecular subgroups: Posterior Fossa A and Posterior Fossa B
- supratentorial ependymoma: 30%
- molecular subgroups: RELA fusion and YAP1 fusion
- spinal cord/canal: 10%
- spinal cord ependymoma: discussed separately
- myxopapillary ependymoma: discussed separately
The remainder of this article concerns itself with intracranial ependymomas.
Epidemiology
Although there is no overall recognized gender predilection when all ependymomas are treated as a group , each subgroup does have different gender and age predilection .
Again, if treated as a group, ependymomas can occur at any age, the posterior fossa tumors tend to present more commonly in the pediatric age group (mean age at diagnosis is 6 years of age), with a smaller second peak for supratentorial tumors around the 3 decade .
Molecular subgroup epidemiology
Each molecular subgroup has different epidemiological profile :
- infratentorial
- Posterior Fossa A
- M:F = 2:1
- age: young children
- Posterior Fossa B
- M:F = 1:1
- age: older children and teenagers
- Posterior Fossa A
- supratentorial
- RELA fusion
- M:F = 2:1
- age: older children
- YAP1 fusion
- M:F = 1:3
- age: <3 years of age
- RELA fusion
Clinical presentation
Clinical presentation can vary according to location. Initial presentation with signs and symptoms of raised intracranial pressure is common, particularly with tumors in the fourth ventricle. Other posterior fossa symptoms including ataxia are also encountered . Supratentorial ependymomas may also present with seizures or focal neurological deficits .
In the infrequent scenario of hemorrhage, the presentation will be hyper-acute.
Associations
- neurofibromatosis type 2 (NF2)
Pathology
Ependymomas are glial tumors with ependymal differentiation which tend to arise within or abutting the ventricular system of the brain or central canal of the spinal cord . Although for many years ependymomas were believed to be tumors arising from dedifferentiated ependymocytes, it now appears fairly certain that in fact, they arise from radial glial stem cells .
Macroscopic appearance
Macroscopically, ependymomas tend to be well defined lobulated grey or tan-colored soft and frond-like tumors which are moderately cellular. They may have focal areas of calcification.
Microscopic appearance
Microscopically, these tumors are characterized by well-differentiated cells. Characteristic features include ependymal rosettes, which are uncommon but pathognomonic and perivascular pseudorosettes which are far more common and seen in most of ependymomas .
Dystrophic calcification, hemorrhage, myxoid degeneration and even rarely metaplasia (bone or cartilage) are sometimes encountered .
Immunohistochemistry
A number of immunohistochemical markers are useful, including :
- glial fibrillary acid protein (GFAP)
- almost always positive in the cytoplasmic process around the perivascular pseudorosettes
- variable elsewhere
- epithelial membrane antigen (EMA)
- positive in the luminal surface of the ependymal rosettes
- positive dot or ring-like perinuclear intracytoplasmic structure (intracytoplasmic microrosette)
- S100: positive
- vimentin: positive
- OLIG2: negative
Classification
Ependymomas are WHO grade II tumors, with more histologically aggressive tumors denoted WHO III (anaplastic ependymoma), although the reliability of histological features in grading these tumors and the prognostic significance is somewhat controversial, and recently there has been a shift towards molecular markers to subdivide ependymomas .
In the current (2016 update) WHO classification of CNS tumors the following entities are accepted as belonging to the ependymoma family of tumors :
- WHO grade I
- subependymoma (discussed separately)
- myxopapillary ependymoma (discussed separately)
- WHO grade II
- ependymoma - 9391/3
- papillary ependymoma
- clear cell ependymoma
- tanycytic ependymoma
- RELA fusion-positive (a new entity in 2016 update)
- ependymoma - 9391/3
- WHO grade III
Note: the 2016 update to WHO classification of CNS tumors has deleted the variant cellular ependymoma as it was felt that it overlapped that of a standard ependymoma too closely .
Molecular subgroup classification
Increasingly, as is the case with many other tumors, molecular classification is proving to be more important than histological classification in predicting treatment response and prognosis. Intracranial ependymomas can be divided into four main groups that correlate largely with location :
- infratentorial
- Posterior Fossa A: H3 K27 trimethylation
- Posterior Fossa B
- supratentorial
- RELA fusion (discussed separately)
- YAP1 fusion
Radiographic features
The majority of intracranial ependymomas (60%) are located in the posterior fossa (infratentorial), usually arising from the lateral recess of the fourth ventricle (molecular subgroup: Posterior Fossa A) and midline inferior floor of the fourth ventricle near the obex (molecular subgroup: Posterior Fossa B) .
The remainder (40%) are located supratentorially and up to half of these are intraparenchymal .
Posterior fossa ependymomas are apt to extend through the foramina of Luschka and Magendie, hence the term plastic ependymoma. This is a characteristic feature and can be seen on both CT and MRI.
Ependymomas are typically heterogeneous masses with areas of necrosis, calcification, cystic change and hemorrhage frequently seen. This results in a heterogeneous appearance on all modalities.
Intraparenchymal lesions (usually supratentorial) are generally large and variable in appearance, ranging from completely solid, enhancing masses to cysts with a mural nodule, or more heterogeneous masses . They are believed to arise from trapping of embryonic rests of ependymal tissue in the developing cerebral parenchyma .
CT
- coarse calcification is common (50%)
- cystic areas (50%)
- solid component iso- to hypodense
- heterogeneous enhancement
- variable hemorrhage
MRI
- T1
- solid portions of ependymoma typically are isointense to hypointense relative to white matter
- T2
- hyperintense to white matter
- more reliable in differentiating tumor margins than non-contrast T1-weighted images (but less reliable than contrast enhanced T1)
- T2* (e.g. SWI)
- foci of blooming from hemorrhage or calcification
- T1 C+ (Gd)
- enhancement present but heterogeneous
- enhancement with gadolinium is useful in differentiating tumor from adjacent vasogenic edema and normal brain parenchyma
- DWI/ADC
- restricted diffusion may be seen in solid components, especially in anaplastic tumor
- diffusion should be interpreted with caution in masses with significant hemorrhage or calcification
- MRS
- Choline peak elevation according to the cellularity of tumor
- NAA peak reduction
- elevated Cho/Cr ratio
- lipid and lactate rise when degeneration occurs
Although it is uncommon when compared to tumors like medulloblastomas, careful examination of the entire neuraxis is required to assess for the presence of CSF seeding.
Treatment and prognosis
A total or partial resection could be attempted +/- irradiation.
Prognosis is, however, relatively poor, which is mainly due to tumors occurring in surgically challenging locations, making complete resection difficult.
Poor prognostic factors include a 4th ventricular location, anaplastic variant and incomplete resection. As such, children have a worse prognosis (both 4th ventricular location and anaplastic variant are more common in children). Overall, the 5-year survival rate in children ranges from 50 to 75% .
Once recurrence has occurred, the prognosis is very poor, with a mortality rate of 90% .
Rarely extraneural metastases may occur, possible sites include the lymph nodes, mediastinum, lungs, pleura, diaphragmatic muscle, retroperitoneum, liver, and bone .
Molecular subgroup prognosis
The molecular subgroup has a have a significant impact on prognosis:
- infratentorial
- Posterior Fossa A: poor
- Posterior Fossa B: good
- supratentorial
- RELA Fusion: poor
- YAP1 Fusion: good
Differential diagnosis
General imaging differential considerations include:
- medulloblastoma
- similar demographic, especially if around the 4 ventricle
- arises from vermis
- less 'plastic', does not tend to extend through foramina
- enhancement more homogenous
- calcification less common
- subependymoma
- tends to occur in older individuals
- usually not enhancing
- choroid plexus papilloma
- in children usually in the trigone of the lateral ventricles
- in adults usually in the fourth ventricle (i.e. opposite to ependymoma)
- more vividly and homogeneously enhancing
- lacks adjacent parenchymal edema
- NB choroid plexus carcinoma can be heterogeneous and invade the brain
- choroid plexus metastasis
- can appear similar
- older individuals, usually with a history of malignancy
- glioblastoma
- difficult to distinguish from intraparenchymal supratentorial ependymoma
- usually older patients
- epicenter usually in the white matter
- central neurocytoma
- usually arises from/in contact with septum pellucidum
- less vivid enhancement
- atypical teratoid/rhabdoid tumor

 Assoziationen und Differentialdiagnosen zu Ependymom:
Assoziationen und Differentialdiagnosen zu Ependymom:







