primäres ZNS-Lymphom
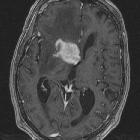





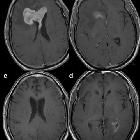

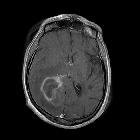






















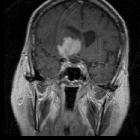









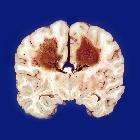
Primary CNS lymphomas (PCNSL) are relatively uncommon tumors, accounting for 2.5% of all brain tumors. By definition, there is no co-existing systemic disease at the time of diagnosis, distinguishing it from CNS involvement from systemic lymphoma (secondary CNS lymphoma).
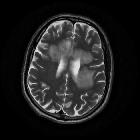
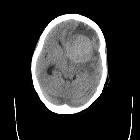
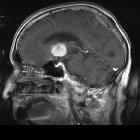
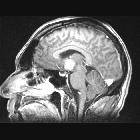
On imaging, PCNSL characteristically is identified as a CT hyperdense enhancing supratentorial mass, with MRI T1 hypointense, T2 iso- to hypointense, vivid homogeneous enhancement and restricted diffusion. Usually, there is relatively little associated vasogenic edema and no central necrosis, although it is important to note that in immunocompromised individuals appearances tend to be more heterogeneous.
Terminology
The current (2016) WHO classification of CNS tumors divides CNS lymphomas into a number of subtypes, based on the cell of origin and histological features. Generally, imaging features are similar and as such this article does not distinguish between these explicitly, grouping them together under the umbrella term primary CNS lymphoma.
A number of subtypes are, however, worth discussing in more detail and these have earned their own articles:
The remainder of this article presents a general discussion of primary CNS lymphoma.
Epidemiology
Typically patients diagnosed with PCNSL are over the age of 50 with a short duration of symptoms (at most a few months) . There is a male predominance of approximately 2:1 .
Historically there has been a strong association with HIV/AIDS and other immunocompromised states and demographics in these patients reflect the underlying condition – see immunodeficiency-associated CNS lymphomas.
More recently, there has been an increase in the incidence of sporadic, non-EBV-associated primary CNS lymphomas in immunocompetent individuals, which is particularly seen in older patients (50-80 years of age) .
Clinical presentation
Patients with primary CNS lymphoma present similarly to patients with other central nervous system masses; symptoms and signs of raised intracranial pressure, focal neurological disorders and seizures.
An important factor to be aware of is the transient but profound response of CNS lymphoma to the use of glucocorticoids (e.g. dexamethasone and prednisolone) which are routinely administered in patients with intracranial mass effect from a tumor and edema. Within a few days of administration of steroids, CNS lymphoma can shrink dramatically due to the combined effect of steroid as a cytotoxic agent (reducing the neoplastic B-cell population) and anti-edema agent (resulting in decreased permeability of capillaries via a variety of mechanisms) . Whatever the underlying mechanism, the result is that administration of steroid prior to biopsy prevents a diagnosis in as many as 50% of cases .
Pathology
The vast majority (>90%) of primary CNS lymphomas are B-cell in origin: diffuse large B-cell lymphoma and high-grade Burkitt-like B-cell lymphoma . Malignant cells tend to accumulate around and within blood vessels. Low-grade tumors are more frequently T-cell in origin .
Location
Primary CNS lymphomas present as solitary (60-70%) or multiple (30-40%) lesions with a predilection for the periventricular white matter, although they can also arise in the cortex or deep grey matter; the latter being more common in low-grade lesions . They are most frequently found in the supratentorial brain (~70%) .
Macroscopic appearance
Macroscopic appearance is variable, ranging from almost indistinguishable from the normal brain to well-circumscribed masses to heterogeneous ill-defined hemorrhagic or necrotic masses .
Microscopic appearance
Primary CNS lymphomas are composed of large quantities of lymphocytes without a particular growth pattern, although there is a predilection for a perivascular distribution, and in many instances infiltration within blood vessels . They may demonstrate areas of necrosis, especially in immunodeficient patients.
Immunophenotype
The exact immunophenotype depends on the tumor type.
Diffuse large B-cell lymphoma, which is the most common, is characterized by immunohistochemical reactivity for CD19, CD20, CD22, CD79a and PAX-5 .
CSF
CSF examination demonstrates elevated protein and decreased glucose. Positive cytology is uncommon (~25%). Positive EBV DNA in CSF is helpful for the diagnosis of lymphoma, particularly in immunocompromised individuals.
Radiographic features
Classic imaging appearance for primary CNS lymphoma is of a CT hyperdense avidly enhancing mass, with T1 hypointense, T2 iso- to hypointense, vivid homogeneous gadolinium-enhancing lesion(s) with restricted diffusion on MRI, and exhibiting subependymal extension and crossing of the corpus callosum.
While this typical pattern is helpful in diagnosis, it is predominantly observed in untreated non-immunocompromised patients. Primary CNS lymphomas in immunocompromised patients (typically HIV/AIDS or post-transplant) may be more heterogeneous tumors, featuring central non-enhancement/necrosis and hemorrhage, although the latter is still uncommon – see immunodeficiency-associated CNS lymphomas .
Typically primary CNS lymphomas are supratentorial (75-85%) and appear as a mass/multiple masses (11-50% ) that are usually in contact with the subarachnoid/ependymal surfaces. Crossing the corpus callosum is not infrequently seen. Enhancement on both CT and MRI is pronounced and usually homogeneous. Even with larger lesions, there is a little mass effect for size and limited surrounding vasogenic edema.
Low-grade tumors differ from the more common high-grade primary CNS lymphomas in several ways :
- deep locations and spinal involvement is more common
- contrast enhancement is absent, irregular or only mild
Disseminated meningeal or intraventricular disease is uncommon. It is seen in ~5% (range 1-7%) of cases at presentation and usually in high-grade cases.
CT
- most lesions are hyperattenuating (70%)
- shows enhancement
- hemorrhage is distinctly uncommon
- often multiple lesions in patients with HIV/AIDS
MRI
Reported signal characteristics include:
- T1: typically hypointense to grey matter
- T1 C+ (Gd)
- typical high-grade tumors show intense homogeneous enhancement while low-grade tumors have absent to moderate enhancement
- peripheral ring enhancement may be seen in immunocompromised patients (HIV/AIDS)
- T2: variable
- majority are iso to hypointense to grey matter
- isointense: 33%
- hypointense: 20% - when present this is a helpful distinguishing feature
- hyperintense: 15-47%, more common in tumors with necrosis
- majority are iso to hypointense to grey matter
- DWI/ADC
- restricted diffusion with ADC values lower than a normal brain, typically between 400 and 600 x 10 mm/s (lower than high-grade gliomas and metastases )
- a number of studies have suggested that the lower the ADC values of a tumor the poorer the response to a tumor and higher likelihood of recurrence
- ADC is particularly useful in assessing response to chemotherapy, with increases in ADC values to above those of normal brain predictive of complete response
- MR spectroscopy
- large choline peak
- reversed choline/creatinine ratio
- markedly decreased NAA
- lactate peak may also be seen
- MR perfusion
- only modest, if any, increase in rCBV (much less marked than in high-grade gliomas, where angiogenesis is a prominent feature )
Nuclear medicine
Thallium-201scintigraphy
- shows increased uptake
PET
fluorine-18FDG PET
- shows increased uptake
carbon-11methionine PET
- shows increased uptake
Treatment and prognosis
Treatment is predominantly with steroids (which can dramatically shrink a tumor due to combined anti-edema and cytotoxic effects) and methotrexate-based chemotherapy . Whole-brain irradiation can also be added, particularly in patients with high-grade tumors, or recurrence .
If a tumor is a low grade (uncommon: see above), then the local treatment with surgical resection and radiotherapy may be effective and long-term survival is possible.
The tumors are often high grade and despite treatment have a poor prognosis. If only surgical resection is performed, then death occurs within a few months. With high dose chemotherapy, the tumor can be significantly reduced in size; however, recurrence is common, with a median survival of around 30 months . Those who are immunocompromised (e.g. HIV positive) have worse outcomes.
Differential diagnosis
For general imaging appearances on CT and MRI consider:
- secondary CNS lymphoma: indistinguishable on imaging, however, it tends to involve more leptomeninges (~2/3 of cases)
- cerebral toxoplasmosis: see toxoplasmosis vs lymphoma
- toxoplasmosis does not exhibit subependymal spread
- more likely to lie in basal ganglia, corticomedullary junction
- CNS lymphoma is thallium/PET avid, whereas toxoplasmosis is not
- butterfly glioma/GBM
- more commonly centrally necrotic
- more commonly demonstrates evidence of hemorrhage
- tumefactive MS/ADEM
- cerebral abscess
- peripheral enhancement of primary CNS lymphoma is thicker
- central restricted diffusion
- neurosarcoidosis

 Assoziationen und Differentialdiagnosen zu primäres ZNS-Lymphom:
Assoziationen und Differentialdiagnosen zu primäres ZNS-Lymphom:







