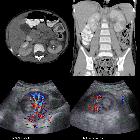
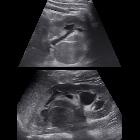
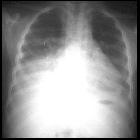
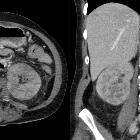
renal cell carcinoma











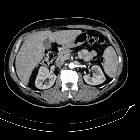





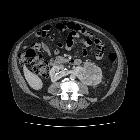


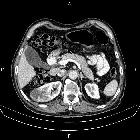


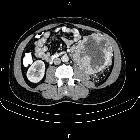

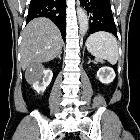

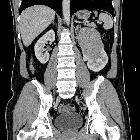










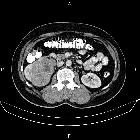



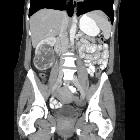
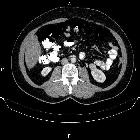





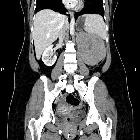













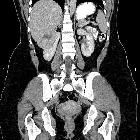











Renal cell carcinomas (RCC) (historically also known as hypernephroma or Grawitz tumor) are primary malignant adenocarcinomas derived from the renal tubular epithelium and are the most common malignant renal tumor. They usually occur in 50-70-year old patients and macroscopic hematuria occurs in 60% of the cases.
On imaging, they have a variety of radiographic appearances, from solid and relatively homogeneous to markedly heterogeneous with areas of necrosis, cystic change, and hemorrhage.
Epidemiology
Patients are typically 50-70 years of age at presentation , with a moderate male predilection of 2:1 .
Renal cell carcinomas are thought to be the 8 most common adult malignancy, representing 2% of all cancers, and account for 80-90% of primary malignant adult renal neoplasms .
Risk factors
- cigarette smoking
- dialysis-related cystic disease
- obesity
- treatment with cyclophosphamide (chemotherapy agent)
- hypertension
- post-renal transplant
Clinical presentation
Presentation is classically described as the triad of:
This triad is however only found in 10-15% of patients , and increasingly the diagnosis is being made on CT for assessment of hematuria alone or as an incidental finding. The majority of cases are sporadic. In contemporary medicine, almost half of all identified renal cell carcinomas are found incidentally on imaging performed for other purposes.
Paraneoplastic syndromes
Around 25% of RCC patients will develop a paraneoplastic syndrome :
- hypercalcemia (20%)
- hypertension (20%)
- polycythemia: from erythropoietin secretion (~5%)
- Stauffer syndrome: hepatic dysfunction not related to metastases
- feminization
- limbic encephalitis
Pathology
Renal cell carcinomas arise from tubular epithelium, and encompasses a number of distinct histological varieties, including :
- clear cell renal carcinoma (conventional): 70-80%
- arises from proximal convoluted tubules
- large uniform cells with clear cytoplasm (thus the name and T2 appearance (see below)
- highly vascular
- subtype: clear cell multilocular renal cell carcinoma
- papillary renal cell carcinoma: 13-20%
- arises from distal convoluted tubules
- can be multifocal and bilateral
- most common form in dialysis-associated RCC
- type I: sporadic, generally good prognosis
- type II: inherited, bilateral and multifocal
- chromophobe renal cell carcinoma: 5%
- arises from intercalated cells of collecting ducts
- similar histologically to renal oncocytomas
- best prognosis
- collecting duct renal cell carcinoma (Bellini duct): <1%
- often younger patients
- worst prognosis
- renal medullary carcinoma: rare
- seen primarily in patients with sickle cell disease or sickle cell trait
- sarcomatoid renal cell carcinoma (sRCC)
- advanced RCC may dedifferentiate into this highly aggressive subtype
Macroscopic appearance
Macroscopically, renal cell carcinomas are variable in appearance, ranging from solid and relatively homogeneous to markedly heterogeneous with areas of necrosis, cystic change, and hemorrhage .
Low grade, smaller tumors typically have a pseudocapsule composed of compressed and ischemic normal renal tissue. The presence of a pseudocapsule is only seen in renal cell carcinomas, renal adenomas, and oncocytomas .
Renal cell carcinoma is one of the more common causes of cannonball metastases to the lung.
Grading
Histological nuclear grading
The most widely used and most predictive grading system for renal cell cancer is the "Fuhrman nuclear grade" which is on a scale of I-IV, where grade I carries the best prognosis and grade IV the worst.
Associations
In some instances RCCs are associated with :
- von Hippel-Lindau syndrome: greater tendency for bilateral RCC as well as a presentation at a younger age; clear cell subtype
- Xp11.2 translocation
- familial clear cell cancer
- tuberous sclerosis
- hereditary renal cell cancer syndromes
Main metastatic sites
The most common sites of metastasis are, in order: the lungs, the bones, lymph nodes, the liver, adrenals, and the brain .
Radiographic features
Imaging is essential in accurately staging renal cell carcinomas (see RCC staging (TNM) and Robson staging) and in operative planning.
Ultrasound
Although ultrasound is very frequently requested to assess the renal tract, it is not as sensitive or specific as CT or MRI. Furthermore, it struggles to accurately locally stage the disease in many instances .
Renal cell carcinoma has a widely varying sonographic appearance. It may appear solid or partially cystic and may be hyper-, iso-, or hypoechogenic to the surrounding renal parenchyma . The tumor pseudocapsule can sometimes be visualized with ultrasound as a hypoechoic halo. Although this is a relatively specific sign, it not particularly sensitive (~20%). Use of harmonic scanning has been reported to increase sensitivity to up to 85% .
Contrast-enhanced ultrasound may typically show a lesion heterogeneously hypervascular in the arterial phase with early washout in the delayed phase.
CT
CT is frequently used to both diagnose and stage renal cell carcinomas.
On non-contrast CT, the lesions are soft tissue attenuation between 20-70 HU . Larger lesions frequently have areas of necrosis. Approximately 30% demonstrate some calcification .
During the corticomedullary phase of enhancement, 25-70 seconds after administration of contrast, renal cell carcinomas demonstrate variable enhancement, usually less than the normal cortex. Small lesions may enhance a similar amount and can be difficult to detect . In general small lesions enhance homogeneously, whereas larger lesions have irregular enhancement due to areas of necrosis. The clear cell subtype may show much stronger enhancement .
The corticomedullary phase is also best for assessing vascular anatomy, both for renal vein involvement, and for arterial variation if partial nephrectomy is being contemplated . Intraluminal growth into the venous circulation, in particular, the renal vein, occurs in 4-15% . The prognosis is significantly worse for those with IVC involvement compared to renal vein involvement alone, making identification on CT important .
The nephrogenic phase (80-180 seconds) is the most sensitive phase for detection of abnormal contrast enhancement.
Excretory phase is of less worth, but important in assessing the collecting system anatomy especially if the candidate is a potential candidate for partial nephrectomy.
Follow-up imaging after treatment is typically done with CT, with dual-phase imaging of the abdomen advocated to maximize the detection of solid organ metastases . Renal cell carcinoma typically causes hypervascular metastases, best appreciated on arterial phase imaging of the upper abdomen.
MRI
MRI is not only excellent at imaging the kidneys and locally staging tumors, but is also able to suggest the likely histology, on the grounds of T2 differences.
- T1: often heterogeneous due to necrosis, hemorrhage, and solid components
- T2: appearances depend on histology
- clear cell RCC: hyperintense
- papillary RCC: hypointense
- T1 C+ (Gd): often shows prompt arterial enhancement
Tumor pseudo capsule, essentially only seen in low-grade renal cell carcinomas, renal adenomas, and oncocytomas appear as a hypointense rim between the tumor and the adjacent normal renal parenchyma .
MRI is also useful for imaging renal vein and IVC tumor thrombus and the rostral extension (important in preoperative planning). The presence of enhancement in the thrombus is able to distinguish between bland and tumor thrombus .
The use of diffusion-weighted sequences has been explored in assisting with characterizing indeterminate small renal lesions, which may be inflammatory or malignant in nature, both exhibit restricted diffusion, albeit the restriction is greater with abscess than tumor.
FDG-PET
Unlike many other malignancies, FDG PET may have limited value in primary renal carcinoma mostly as result of physiological excretion of FDG from the kidneys, which decreases contrast between renal lesions and normal tissue, and may obscure or mask the lesions of the kidneys . However certain publications suggest it can effectively be used for postoperative surveillance and restaging as an adjunct when conventional imaging is not conclusive and in some situations such as differentiating benign or bland emboli from tumor emboli .
Treatment and prognosis
Treatment of renal cell carcinomas is usually with radical nephrectomy if feasible. However, in elderly patients or those with co-morbidities, and especially those with smaller tumors suggestive of papillary histology (see MRI findings above) then organ-sparing treatment can be entertained. This ranges from adrenal sparing nephrectomy to partial nephrectomy, performed both open or laparoscopically. Additionally, percutaneous radiofrequency or cryoablation (typically under CT guidance), which can be carried out with only local anesthetic and sedation, has been introduced in selected cases .
Prognosis can be variable depending on both on histological subtype and stage.
The papillary variant carries the best prognosis (5-year survival of 90%), followed by clear cell (conventional) RCC (5-year survival 70%) while collecting duct subtype carries the worst .
As far as the effects of tumor stage (see renal cell carcinoma staging) are concerned, there is a dramatic difference between stage I and IV tumors:
- stage I: 90% 5-year survival
- stage II: 50% 5-year survival
- stage III: 30% 5-year survival
- stage IV: 5% 5-year survival
Approximately one-third of the newly diagnosed cases of the RCC have metastatic disease at the time of initial presentation (synchronous metastases) .
Differential diagnosis
The broad differential is essentially that of all renal masses, particularly other renal tumors, and most commonly includes:
- renal tumors
- renal adenoma: should be considered small, early renal cell carcinomas
- renal oncocytoma: particularly for chromophobe type
- angiomyolipoma (AML):
- usually has a large component of fat
- 4-5% have little or no fat however
- renal metastasis
- renal lymphoma
- solitary fibrous tumor of the kidney: rare
- multilocular cystic nephroma: indistinguishable from multilocular clear cell carcinoma
- renal pseudotumors
- hemorrhagic or complex renal cyst: see Bosniak classification
- renal abscess/pyelonephritis/lobar nephronia
- renal infarct
- hypertrophied column of Bertin
- direct extension of neighboring tumors

 Assoziationen und Differentialdiagnosen zu Nierenzellkarzinom:
Assoziationen und Differentialdiagnosen zu Nierenzellkarzinom: