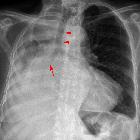
congenital cystic adenomatoid malformation

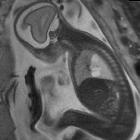








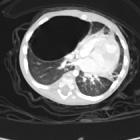

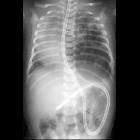













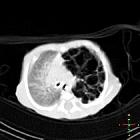



Congenital pulmonary airway malformations (CPAM) are multicystic masses of segmental lung tissue with abnormal bronchial proliferation. CPAMs are considered part of the spectrum of bronchopulmonary foregut malformations.
Terminology
Until recently they were described as congenital cystic adenomatoid malformations (CCAM).
Epidemiology
They account for ~25% of congenital lung lesions. The estimated incidence is approximately 1:1500-4000 live births and there is a male predominance.
Clinical presentation
The diagnosis is usually either made on antenatal ultrasound, or in the neonatal period on the investigation of progressive respiratory distress . If large, they may cause pulmonary hypoplasia, with resultant poor prognosis.
In cases where the abnormality is small, the diagnosis may not be made for many years or even until adulthood. When it does become apparent, it is usually as a result of recurrent chest infection .
Pathology
The condition results from failure of normal bronchoalveolar development with a hamartomatous proliferation of terminal respiratory units in a gland-like pattern (adenomatoid) without proper alveolar formation.
Histologically, they are characterized by adenomatoid proliferation of bronchiole-like structures and macro- or microcysts lined by columnar or cuboidal epithelium and absence of cartilage and bronchial glands.
These lesions have intracystic communications and, unlike bronchogenic cysts, can also have a connection to the tracheobronchial tree.
Subtypes
Five subtypes are currently classified, mainly according to cyst size:
- type I
- most common: 70% of cases
- large cysts
- one or more dominant cysts: 2-10 cm in size
- may be surrounded by smaller cysts
- type II
- 15-20% of cases
- cysts are <2 cm in diameter
- associated with other abnormalities
- type III
- ~10% of cases
- microcysts: <5 mm in diameter
- typically involves an entire lobe
- has a poorer prognosis
- type IV
- unlined cyst
- typically affects a single lobe
- indistinguishable from type I on imaging
- type 0
- very rare, lethal postnatally
- acinar dysgenesis or dysplasia
- represents global arrest of lung development
Location
Lesions are usually unilateral and involve a single lobe. Although there is no well-documented lobar predilection, they appear less frequently in the middle lobe .
Associations
- hybrid lesion: i.e. CPAM and pulmonary sequestration
- renal agenesis
- polyhydramnios
- hydrops fetalis
- lung malignancy
- ~10% of pediatric lung cancers have a history of CPAM
- bronchoalveolar carcinoma associated with type 1 CPAM
- pleuropulmonary blastoma associated with type 4 CPAM
Radiographic features
The appearance of CPAMs will vary depending on the type.
Antenatal ultrasound
CPAM appears as an isolated cystic or solid intrathoracic mass. A solid thoracic mass is usually indicative of a type III CPAM and is typically hyperechoic. There can be a mass effect where the heart may appear displaced to the opposite side. Alternatively, the lesion may remain stable in size, or even regress .
Hydrops fetalis and polyhydramnios may develop and may be detected on ultrasound as ancillary sonographic features .
Plain radiograph
Chest radiographs in type I and II CPAMs may demonstrate a multicystic (air-filled) lesion. Large lesions may cause a mass effect with resultant mediastinal shift, depression, and even inversion of the diaphragm. In the early neonatal period, the cysts may be completely or partially fluid-filled, in which case the lesion may appear solid or with air-fluid levels. Lesions may change in size on interval imaging (expand from collateral ventilation via pores of Kohn). Type III lesions appear solid.
CT
CT has a number of roles in the management of CPAMs. First, it more accurately delineates the location and extent of the lesion. Secondly, and most important in surgical candidates, CT angiography is able to identify systemic arterial supply if present.
Appearance reflects the underlying type, and a type III lesion can appear as a consolidation.
Treatment and prognosis
There can be a wide spectrum of prognosis.
Surgery (elective lobectomy) is the treatment of choice in symptomatic patients, both in those presenting early with respiratory compromise and in those presenting later with recurrent infections . Type I lesions have the best prognosis.
In the setting of a small stable asymptomatic lesion, surgical excision is more controversial. Advocates for excision quote the reported risk of developing malignancies within the lesion (see above). An alternative approach is to watch and wait. There are reports of spontaneous regression, particularly in those serially followed up on antenatal ultrasound .
Complications
Potential postnatal complications include:
- recurrent pneumothorax
- hemopneumothorax
- pyopneumothorax
- possible incidence of certain malignancies, which include :
Potential in utero complications include:
- hydrops fetalis may rarely develop when there is severe compression of the fetal heart or great vessels
- compression of the normal fetal lung can also rarely cause pulmonary hypoplasia
Differential diagnosis
General imaging differential considerations include:
- bronchogenic cyst
- Unilocular
- does not usually communicate with the bronchial tree, and are therefore typically not air-filled
- pulmonary sequestration
- systemic arterial supply
- hybrid lesions may present with both CPAM and sequestration features (see above)
- congenital diaphragmatic herniation
- bowel loops within a hemithorax
- congenital lobar emphysema (congenital lobar overinflation)
- hyperlucent and hyperinflated lung segment
- no cystic or solid components
- localized congenital cystic bronchiectasis
For type I lesions on CT also consider:
- cicatrisation collapse with scarring and traction bronchiectasis
Siehe auch:
- Pneumothorax
- Lungenkarzinom
- Lungensequester
- Bronchogene Zyste
- Hydrops fetalis
- Rhabdomyosarkom
- Herzfehler
- Lungenhypoplasie
- In Situ Adenokarzinom der Lunge
- Polyhydramnion
- einseitige Nierenagenesie
- kongenitales lobäres Emphysem
- Pleuro-pulmonales Blastom
- kongenitale Zwerchfellhernie
- congenital cystic bronchiectasis
- hybrid lesion: CCAM - pulmonary sequestration
- congenital cystic adenomatoid malformation (CCAM) / congenital pulmonary airway malformation (CPAM)
- bronchopulmonale Vordarmmalformation
- pores of Kohn
und weiter:
- Pneumatozele
- obstetric curriculum
- Kavernöse Lungenläsionen
- multiple zystische Lungenherde
- fetal pleural effusion
- interstitielles Lungenemphysem
- pulmonary cavity (mnemonic)
- neonatal chest radiograph in the exam setting
- sonographic values in obstetrics and gynaecology
- Lungensequester extralobulär
- echogenic fetal lung lesions
- PIE
- zystische Lungenläsionen bei Kindern
- cytic lung lesions - paediatric
- hybrid lesion : paediatric chest
- intralobar pulmonary sequestration
- Pneumothorax und Fliegen
- CCAM (Zystisch adenomatoide Malformation der Lunge) im fetalen MRT
- kindliche Lungenläsionen
- Prune-Belly Syndrome with congenital cystic adenomatoid malformation
- Acute Respiratory Distress Syndrome neonatal
- Zwerchfelldefekte
- intrapulmonale bronchogene Zyste

 Assoziationen und Differentialdiagnosen zu kongenitale pulmonale Atemwegsmalformation (CPAM):
Assoziationen und Differentialdiagnosen zu kongenitale pulmonale Atemwegsmalformation (CPAM):