Chordom



























Chordomas are uncommon malignant tumors of the axial skeleton that account for 1% of intracranial tumors and 4% of all primary bone tumors.
They originate from embryonic remnants of the primitive notochord (earliest fetal axial skeleton, extending from the Rathke's pouch to the tip of the coccyx). Since chordomas arise in bone, they are usually extradural and result in local bone destruction. They are locally aggressive but uncommonly metastasize.
Epidemiology
Chordomas can occur at any age but are usually seen in adults (30-70 years). Those located in the spheno-occipital region most commonly occur in patients 20-40 years of age, whereas sacrococcygeal chordomas are typically seen in a slightly older age group (peak around 50 years ). They are commonly found in Caucasians .
Clinical presentation
They are slow growing tumors and usually present due to mass effect on adjacent structures (brainstem, cranial nerves, nasopharynx, spinal cord), or as a palpable mass (e.g. sacrococcygeal chordoma) .
Pathology
Macroscopically, chordomas present as firm masses. Fluid and gelatinous mucoid substance (associated with recent and old hemorrhage) and necrotic areas are found within these tumors. In some patients, calcification and sequestered bone fragments are found as well. The variety of these components may explain the signal heterogeneity observed on MRI.
Microscopically, chordomas are characterized by physaliphorous cells. These tumors are often poorly marginated and microscopic distal extension of tumor cells likely explains the frequency of recurrences.
Three subtypes are recognized :
- conventional chordoma (most common)
- chondroid chordoma (best prognosis)
- dedifferentiated chordoma (least common, worst prognosis)
True malignant forms of chordomas occasionally have areas of typical chordoma as well as undifferentiated areas, most often fibrosarcoma. The prognosis in such cases is poor.
Location
Chordomas are found along the axial skeleton and a relatively evenly distributed among three locations:
- sacrococcygeal: 30-50%
- spheno-occipital: 30-35%
- vertebral body: 15-30%
Sacrococcygeal
Sacrococcygeal is the most common location, accounting for approximately 30-50% of all chordomas and commonly involving the fourth and fifth sacral segments . In this location, a male predilection has been reported (M:F ratio of 2:1), and the tumor may be particularly large at presentation .
Chordoma is the most common primary malignant sacral tumor .
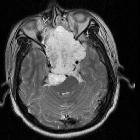
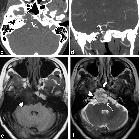
Spheno-occipital
The clival region is the second most common location, accounting for 30-35% of cases . Typically the mass projects posteriorly at midline, indenting the pons; this characteristic appearance has been termed the so-called thumb sign. In contrast to sacrococcygeal tumors, there is no recognized gender difference.
Vertebral bodies
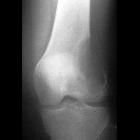
Chordomas of the vertebral bodies are rare, but nonetheless, after lymphoproliferative tumors, are the second most common primary malignancy of the spine in adults .
They most commonly involve cervical (particularly C2), followed by lumbar, and then the thoracic spine. They often extend across the intervertebral disc space, involving more than one vertebral segment. They may extend into the epidural space compressing the spinal cord, or along the nerve roots enlarging the neural foramen.
Radiographic features
MRI and CT scan have complementary roles in tumor evaluation. CT evaluation is needed to assess the degree of bone involvement and to detect patterns of calcification within the lesion. MRI provides excellent anatomical delineation of adjacent structures and is able to characterize the signal of the lesion usually allowing for a confident preoperative diagnosis. MRI is, however, limited in its ability to evaluate calcification and the precise involvement of skull base osteolysis less well than CT, especially for skull base foramina.
CT
- centrally located
- well-circumscribed
- destructive lytic lesion, sometimes with marginal sclerosis
- expansile soft-tissue mass
- usually hyper-attenuating relative to adjacent brain; however, inhomogenous areas may be seen due to necrosis or hemorrhage
- soft-tissue mass is often disproportionately large relative to the bony destruction
- irregular intratumoral calcifications (thought to represent sequestra of normal bone rather than dystrophic calcifications)
- moderate to marked enhancement
MRI
- T1
- intermediate to low-signal intensity
- small foci of hyperintensity (intratumoral hemorrhage or a mucus pool)
- T2: most exhibit very high signal
- T1 C+ (Gd)
- heterogeneous enhancement with a honeycomb appearance corresponding to low T1 signal areas within the tumor
- greater enhancement has been associated with poorer prognosis
- SWI/GE: variable intralesional hemorrhage, suggested by the presence of blooming artefact
- DWI/ADC
- conventional chordoma: 1474 ± 117 x 10 mm/s
- dedifferentiated chordoma: 875 ± 100 x 10 mm/s
Bone scan
- variable
Angiography
Although angiography is useful to assess vascular encasement and displacement, chordomas usually do not have significant tumoral vascularity .
Treatment and prognosis
Traditionally, surgical resection has been the first line of treatment when feasible, with radiotherapy offered for recurrent cases. Some advocate the combination of radiation therapy and complete or subtotal surgical resection for select patients . Percutaneous radiofrequency ablation has been trialled as an adjunct therapy . Recurrence, including seeding along the operative tract, is common.
Metastatic spread of chordomas is observed in 7-14% of patients and includes nodal, pulmonary, bone, cerebral or abdominal visceral involvement, predominantly from massive tumors .
Prognosis is typically poor due to the locally aggressive nature of these tumors, with overall 10-year survival of approximately 40%.
Histological subtype also has a substantial impact on prognosis with chondroid chordoma having the best prognosis and dedifferentiated chordoma the worst prognosis; the more common conventional chordoma having intermediate prognosis .
Differential diagnosis
For clival/spheno-occipital lesions differentials to consider include:
- chondrosarcoma of skull base
- plasmacytoma
- meningioma of skull base
- pituitary macroadenoma
- ecchordosis physaliphora
- benign notochordal cell tumor
For vertebral lesions, consider:
- chondrosarcoma
- neural arch > vertebral body
- thoracic spine is the most commonly involved spinal region
- chondroid matrix (rings & arcs)
- similar MRI appearance to chordomas (low to intermediate signal intensity on T1, hyperintense on T2, enhances)
- higher ADC values: 2051 ± 261 x 10 mm/s
- giant cell tumor
- F>M
- location: sacrum > thoracic spine > cervical spine > lumbar spine
- no mineralized matrix
- heterogeneous intermediate to hyperintense T2 signal
- spinal metastases
- hypointense on T1; variably hyperintense on T2
- often multiple, involving vertebral bodies and posterior elements
- plasmacytoma
- destructive vertebral body lesion (similar appearance to lytic metastases)
- spinal lymphoma
- multifocal disease
- heterogenous T2 signal
Siehe auch:
- Meningeom
- Raumforderungen des Clivus
- Chondrosarkom
- Makroadenom Hypophyse
- Tumoren Os sacrum
- Ecchordosis physaliphora
- Multiples Myelom
- Riesenzelltumor
- Steißbeinteratom
- Chordom am Clivus
- spinale Metastasen
- Chondrosarkom der Schädelbasis
- sacrococcygeales Chordom
- metastases from chordoma
- spinales Lymphom
und weiter:
- Tumoren der Schädelkalotte
- Cholesteatom
- Tuberöse Sklerose
- Tumoren der Hypophysenregion
- Tumor Kleinhirnbrückenwinkel
- präsakrale Tumoren
- Ästhesioneuroblastom
- neuroradiologisches Curriculum
- erworbenes Cholesteatom
- Tumoren des hinteren Mediastinums
- spinal myxopapillary ependymoma
- Cholesteatom des äußeren Gehörgangs
- posterior mediastinal masses
- Karzinom des Nasopharynx
- radiologisches muskuloskelettales Curriculum
- spinales Ependymom
- Knochentumoren
- Cavum trigeminale
- spinales Ependymom des Filum terminale
- arrested pneumatization of skull base
- Chorda dorsalis
- Erweiterung des interpedunculären Abstands
- myxoides Chondrosarkom
- prevertebral space
- primary tumours of the spine
- Parachordom
- benigner notochordaler Tumor
- metastases from a sacral chordoma
- arrested pneumatisation of the skull base
- lumbar spine chordoma
- Pyramidenspitzen-Osteomyelitis

 Assoziationen und Differentialdiagnosen zu Chordom:
Assoziationen und Differentialdiagnosen zu Chordom: