Subarachnoidalblutung

















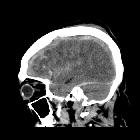

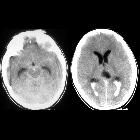




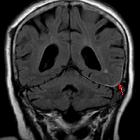









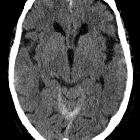










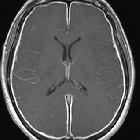
Subarachnoid hemorrhage (SAH) is a type of extra-axial intracranial hemorrhage and denotes the presence of blood within the subarachnoid space.
Epidemiology
Patients tend to be older middle age, typically less than 60 years old . Subarachnoid hemorrhage accounts for 3% of stroke and 5% of stroke deaths .
Risk factors
Risk factors include :
- family history
- hypertension
- heavy alcohol consumption
- abnormal connective tissue
- female gender: ~1.5x baseline risk
- African descent: ~2x baseline risk
- Japanese or Finnish descent
Clinical presentation
Patients typically present with a thunderclap headache, described as a sudden-onset headache that is the worst headache of their life. It is often associated with photophobia and meningism. Focal neurological deficits often present either at the same time as a headache or soon thereafter .
In a substantial number of patients (almost half ), it is associated with collapse and decreased or loss of consciousness, even in those patients who subsequently regain consciousness and have a good grade.
Patients can be graded into five groups based on their clinical presentation, using the commonly employed Hunt and Hess grading system, which is predictive of outcome.
Pathology
Three distinct patterns of subarachnoid hemorrhage have been described each with their own etiology and treatment/prognostic implications :
Etiology
Causes include :
- trauma
- spontaneous
- ruptured berry aneurysm: 85%
- perimesencephalic hemorrhage: 10%
- arteriovenous malformation
- cerebral amyloid angiopathy
- ruptured mycotic aneurysm
- reversible cerebral vasoconstriction syndrome
- dural arteriovenous fistula
- spinal arteriovenous malformation
- venous infarction
- intradural arterial dissection
- pituitary apoplexy
- sympathomimetic drugs (eg. cocaine)
- cerebral vasculitis
- anticoagulation therapy
Radiographic features
Although MRI is thought to be more sensitive, CT is frequently performed first due to wider availability. As well as being more sensitive to hemorrhage, MRI has greater sensitivity to the wide range of causative lesions.
A description of the radiographic features of each causative underlying lesion is clearly beyond the scope of this article; these are discussed separately (see above).
CT
The sensitivity of CT to the presence of subarachnoid blood is strongly influenced by both the amount of blood and the time since the hemorrhage.
The diagnosis is suspected when a hyperdense material is seen filling the subarachnoid space. Most commonly this is apparent around the circle of Willis, on account of the majority of berry aneurysms occurring in this region (~65%), or in the Sylvian fissure (~30%) .
Small amounts of blood can sometimes be appreciated pooling in the interpeduncular fossa, appearing as a small hyperdense triangle, or within the occipital horns of the lateral ventricles .
Subarachnoid hemorrhages are grouped into four categories according to the amount of blood on unenhanced CT by the Fisher scale. This scale has been updated to the modified Fisher scale, which more accurately correlates the risk of vasospasm.
MRI
MRI is sensitive to subarachnoid blood and is able to visualize it well in the first 12 hours typically as a hyperintensity in the subarachnoid space on FLAIR .
Susceptibility weighted sequences are also exquisitely sensitive to blood products.
MR angiography and MR venography are also able to detect a causative aneurysm or another source of bleeding, although in general MRI suffers from poor availability (compared to CT), longer scan times and greater difficulty in transferring and looking after patients who are often unstable and intubated.
In aneurysm-associated subarachnoid hemorrhage, diffusion weighted imaging may demonstrate early ischemic changes (within 0-3 days) in more than half of all patients . Additional delayed ischemia detectable with DWI, associated with vasospasm developing 4-21 days after ictus, may develop in about half of all patients .
DSA
Digital subtraction catheter angiography remains the gold standard for diagnosis and characterization of vascular abnormalities and in many centers, even if the causative lesion is identified on MRA or CTA and it is thought to require surgical management, a catheter study is carried out.
The benefit of DSA is two-fold:
Additionally, depending on the cause, endovascular therapy (e.g. aneurysm coiling) may be appropriate.
Treatment and prognosis
Treatment will vary according to the underlying cause, however, regardless of the source of subarachnoid blood, a number of treatment principles and potential complications are encountered:
- elevated intracranial pressure
- often require ICP monitoring
- serial non-invasive screening possible in equivocal cases using sonographic indices of elevated ICP
- hydrocephalus may require extraventricular drain placement
- often require ICP monitoring
- cerebral vasospasm causing delayed cerebral ischemia
- triple H therapy (Haemodilution, Hypertension, Hypervolaemia)
- calcium channel blockers (e.g. nimodipine)
- endovascular intervention (e.g. intra-arterial delivery of vasodilating agents (such as NO) and/or balloon angioplasty)
- hyponatremia
- coronary spasm
- neurogenic pulmonary edema
- pulseless electrical activity (PEA)
Prognosis varies greatly depending on:
- cause of subarachnoid hemorrhage
- grade of subarachnoid hemorrhage
- presence of other injuries/pathologies/co-morbidities
A small amount of traumatic subarachnoid hemorrhage or small perimesencephalic blood has an excellent prognosis with little if any significant long-term sequelae. A grade V aneurysmal subarachnoid, on the other hand, has a dismal prognosis, despite aggressive treatment.
Differential diagnosis
It is important to realize that apparent hyperdensity in the subarachnoid space is not pathognomonic of subarachnoid hemorrhage. Other diagnostic possibilities include:
- pseudosubarachnoid hemorrhage
- bacterial meningitis
- tuberculous meningitis
- granulomatous meningitis

 Assoziationen und Differentialdiagnosen zu Subarachnoidalblutung:
Assoziationen und Differentialdiagnosen zu Subarachnoidalblutung: